23.14: Interesting and Useful Amines
- Page ID
- 30955
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
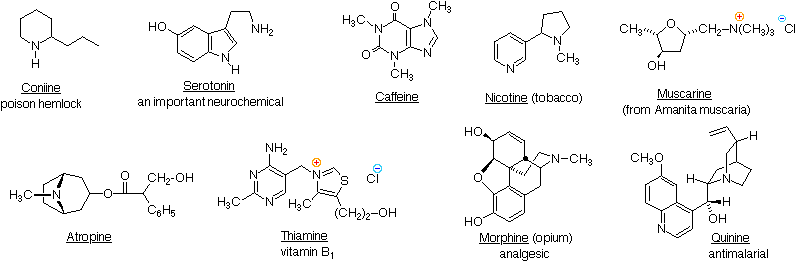
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Nature abounds with nitrogen compounds, many of which occur in plants and are referred to as alkaloids. Structural formulas for some representative alkaloids and other nitrogen containing natural products are displayed below, and we can recognize many of the basic structural features listed above in their formulas. Thus, Serotonin and Thiamine are 1º-amines, Coniine is a 2º-amine, Atropine, Morphine and Quinine are 3º-amines, and Muscarine is a 4º-ammonium salt.
The reader should be able to recognize indole, imidazole, piperidine, pyridine, pyrimidine & pyrrolidine moieties among these structures. These will be identified by pressing the "Show Structures" button under the diagram.
Nitrogen atoms that are part of aromatic rings , such as pyridine, pyrrole & imidazole, have planar configurations (sp2 hybridization), and are not stereogenic centers. Nitrogen atoms bonded to carbonyl groups, as in caffeine, also tend to be planar. In contrast, atropine, coniine, morphine, nicotine and quinine have stereogenic pyramidal nitrogen atoms in their structural formulas (think of the non-bonding electron pair as a fourth substituent on a sp3 hybridized nitrogen). In quinine this nitrogen is restricted to one configuration by the bridged ring system. The other stereogenic nitrogens are free to assume two pyramidal configurations, but these are in rapid equilibrium so that distinct stereoisomers reflecting these sites cannot be easily isolated.
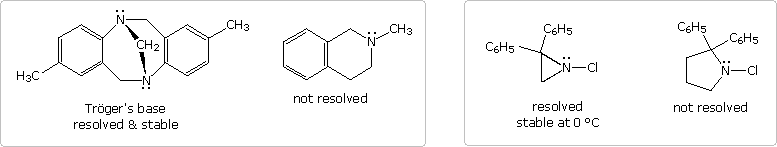
It should be noted that structural factors may serve to permit the resolution of pyramidal chiral amines. Two examples of such 3º-amines, compared with similar non-resolvable analogs, are shown in the following diagram. The two nitrogen atoms in Trögers base are the only stereogenic centers in the molecule. Because of the molecule's bridged structure, the nitrogens have the same configuration and cannot undergo inversion. The chloro aziridine can invert, but requires a higher activation energy to do so, compared with larger heterocyclic amines. It has in fact been resolved, and pure enantiomers isolated. An increase in angle strain in the sp2-hybridized planar transition state is responsible for the greater stability of the pyramidal configuration. The rough estimate of angle strain is made using a C-N-C angle of 60º as an arbitrary value for the three-membered heterocycle.
Of course, quaternary ammonium salts, such as that in muscarine, have a tetrahedral configuration that is incapable of inversion. With four different substituents, such a nitrogen would be a stable stereogenic center.

Amines as Antidepressants
Antidepressant drugs act by one or more of the following stimulation type mechanisms:
- Increase release of norepinephrine:Amphetamines and electroconvulsive therapy act by this mechanism. Amphetamines mimic norepinephrine.
- Prevent inactivation of norepinephrine:Monoamine oxidase (MAO) inhibitors are thought to act as antidepressant agents in part by preventing the breakdown and inactivation of norepinephrine.
- Prevent the re uptake of norepinephrine:The action of norepinephrine at the receptor site is terminated by the re uptake of norepinephrine by the neuron from which it was originally released.
Tricyclic Antidepressants
The tricyclic antidepressants are the most effective drugs presently available for the treatment of depression. These act by increasing the release of norepinephrine. Amphetamine and cocaine can also act in this manner. Imipramine, amitriptylin, and other closely related drugs are among the drugs currently most widely used for the treatment of major depression.
- imipramine (Tofranil)
- desipramine (Norpramin)
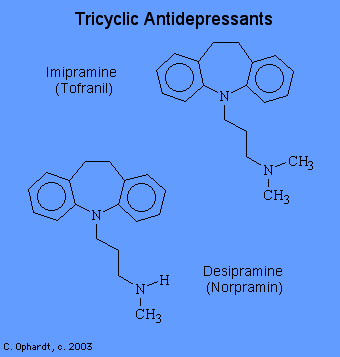
The activity of the tricyclic drugs depends on the central ring of seven or eight atoms which confers an angled or twisted conformation. The side chain must have at least 2 carbons although 3 appear to be better. The amine group may be either tertiary or secondary. All tricyclic antidepressants block the re-uptake of norepinephrine at nerve terminals. However, the potency and selectivity for the inhibition of the uptake of norepinephrine, serotonin, and dopamine vary greatly among the agents. The tertiary amine tricyclics seem to inhibit the serotonin uptake pump, whereas the secondary amine ones seem better in switching off the NE pump. For instance, imipramine is a potent and selective blocker of serotonin transport, while desipramine inhibits the uptake of norepinephrine.
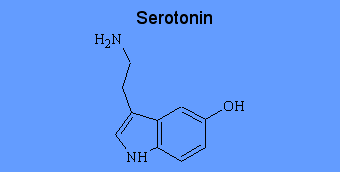
Serotonin
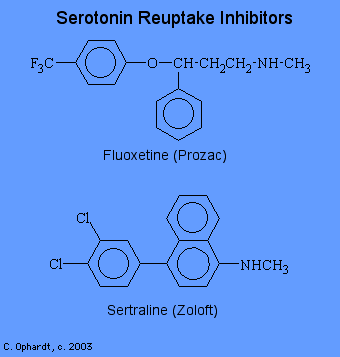
Serotonin (5-hydroxytryptamine or 5-HT) is a monoamine neurotransmitter found in cardiovascular tissue, in endothelial cells, in blood cells, and in the central nervous system. The role of serotonin in neurological function is diverse, and there is little doubt that serotonin is an important CNS neurotransmitter. Although some of the serotonin is metabolized by monoamine oxidase, most of the serotonin released into the post-synaptic space is removed by the neuron through a re uptake mechanism inhibited by the tricyclic antidepressants and the newer, more selective antidepressant re uptake inhibitors such as fluoxetine and sertraline.
Selective Serotonin Reuptake Inhibitors
In recent years, selective serotonin reuptake inhibitors have been introduced for the treatment of depression. Prozac is the most famous drug in this class. Clomiprimine, fluoxetine (Prozac), sertraline and paroxetine selectively block the re uptake of serotonin, thereby increasing the levels of serotonin in the central nervous system. Note the similarities and differences between the tricyclic antidepressants and the selective serotonin re uptake inhibitors. Clomipramine has been useful in the treatment of obsessive-compulsive disorders.
Monoamine Oxidase Inhibitors
Monoamine oxidase (MAO) causes the oxidative deamination of norephinephrine, serotonin, and other amines. This oxidation is the method of reducing the concentration of the neurotransmitter after it has sent the signal at the receptor site. A drug which inhibits this enzyme has the effect of increasing the concentration of the norepinephrine which in turn causes a stimulation effect. Most MAO inhibitors are hydrazine derivatives. Hydrazine is highly reactive and may form a strong covalent bond with MAO with consequent inhibition for up to 5 days.
These drugs are less effective and produce more side effects than the tricyclic antidepressants. For example, they lower blood pressure and were at one time used to treat hypertension. Their use in psychiatry has also become very limited as the tricyclic antidepressants have come to dominate the treatment of depression and allied conditions. Thus, MAOIs are used most often when tricyclic antidepressants give unsatisfactory results.
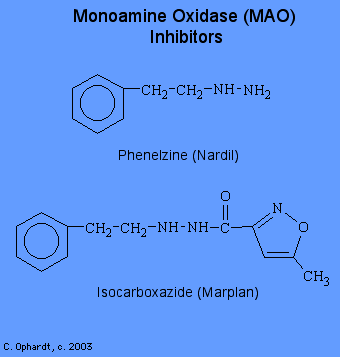
Phenelzine is the hydrazine analog of phenylethylamine, a substrate of MAO. This and several other MAOIs, such as isocarboxazide, are structurally related to amphetamine and were synthesized in an attempt to enhance central stimulant properties.
- phenelzine (Nardil)
- isocarboxazid (Marplan)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Jim Clark (Chemguide.co.uk)