10.6: Lipids—Part 2
- Page ID
- 30465
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Fatty acids are merely carboxylic acids with long hydrocarbon chains. The hydrocarbon chain length may vary from 10-30 carbons (most usual is 12-18). The non-polar hydrocarbon alkane chain is an important counter balance to the polar acid functional group. In acids with only a few carbons, the acid functional group dominates and gives the whole molecule a polar character. However, in fatty acids, the non-polar hydrocarbon chain gives the molecule a non-polar character.
Introduction
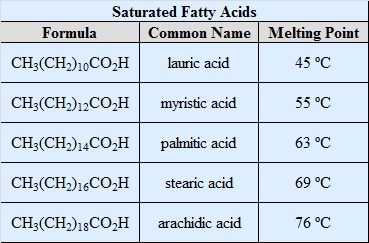
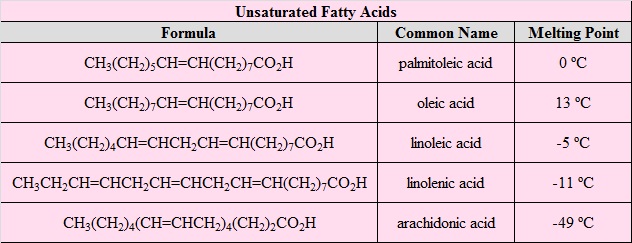
The most common fatty acids are listed. Note that there are two groups of fatty acids--saturated and unsaturated. Recall that the term unsaturated refers to the presence of one or more double bonds between carbons as in alkenes. A saturated fatty acid has all bonding positions between carbons occupied by hydrogens.
The melting points for the saturated fatty acids follow the boiling point principle observed previously. Melting point principle: as the molecular weight increases, the melting point increases. This observed in the series lauric (C12), palmitic (C16), stearic (C18). Room temperature is 25oC, Lauric acid which melts at 44o is still a solid, while arachidonic acid has long since melted at -50o, so it is a liquid at room temperature.
Table 1: Common Fatty Acids
Melting Points of Saturated vs. Unsaturated Fatty Acids
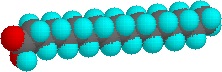
Note that as a group, the unsaturated fatty acids have lower melting points than the saturated fatty acids. The reason for this phenomenon can be found by a careful consideration of molecular geometries. The tetrahedral bond angles on carbon results in a molecular geometry for saturated fatty acids that is relatively linear although with zigzags.
|
|
| |
| Stearic acid | Oleic aci |
This molecular structure allows many fatty acid molecules to be rather closely "stacked" together. As a result, close intermolecular interactions result in relatively high melting points.
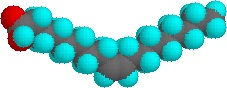
On the other hand, the introduction of one or more double bonds in the hydrocarbon chain in unsaturated fatty acids results in one or more "bends" in the molecule. The geometry of the double bond is almost always a cis configuration in natural fatty acids. and these molecules do not "stack" very well. The intermolecular interactions are much weaker than saturated molecules. As a result, the melting points are much lower for unsaturated fatty acids.
| | |||||
| Fat or Oil | | | |||
| Palmitic | Stearic | Oleic | Linoleic | Other | |
| Animal Origin | |||||
| Butter | 29 | 9 | 27 | 4 | 31 |
| Lard | 30 | 18 | 41 | 6 | 5 |
| Beef | 32 | 25 | 38 | 3 | 2 |
| Vegatable Origin | |||||
| Corn oil | 10 | 4 | 34 | 48 | 4 |
| Soybean | 7 | 3 | 25 | 56 | 9 |
| Peanut | 7 | 5 | 60 | 21 | 7 |
| Olive | 6 | 4 | 83 | 7 | - |
Saturated vs. Unsaturated Fatty Acids in Fats and Oils
The triesters of fatty acids with glycerol (1,2,3-trihydroxypropane) compose the class of lipids known as fats and oils. These triglycerides (or triacylglycerols) are found in both plants and animals, and compose one of the major food groups of our diet. Triglycerides that are solid or semisolid at room temperature are classified as fats, and occur predominantly in animals. Those triglycerides that are liquid are called oils and originate chiefly in plants, although triglycerides from fish are also largely oils. Some examples of the composition of triglycerides from various sources are given in the following table.
| Saturated Acids (%) | Unsaturated Acids (%) | |||||||
| Source | C10 | C12 | C14 | C16 | C18 | C18 | C18 | C18 |
| Animal Fats | ||||||||
| butter | 15 | 2 | 11 | 30 | 9 | 27 | 4 | 1 |
| lard | - | - | 1 | 27 | 15 | 48 | 6 | 2 |
| human fat | - | 1 | 3 | 25 | 8 | 46 | 10 | 3 |
| herring oil | - | - | 7 | 12 | 1 | 2 | 20 | 52 |
| Plant Oils | ||||||||
| coconut | - | 50 | 18 | 8 | 2 | 6 | 1 | - |
| corn | - | - | 1 | 10 | 3 | 50 | 34 | - |
| olive | - | - | - | 7 | 2 | 85 | 5 | - |
| palm | - | - | 2 | 41 | 5 | 43 | 7 | - |
| peanut | - | - | - | 8 | 3 | 56 | 26 | 7 |
| safflower | - | - | - | 3 | 3 | 19 | 76 | - |
As might be expected from the properties of the fatty acids, fats have a predominance of saturated fatty acids, and oils are composed largely of unsaturated acids. Thus, the melting points of triglycerides reflect their composition, as shown by the following examples. Natural mixed triglycerides have somewhat lower melting points, the melting point of lard being near 30 º C, whereas olive oil melts near -6 º C. Since fats are valued over oils by some Northern European and North American populations, vegetable oils are extensively converted to solid triglycerides (e.g. Crisco) by partial hydrogenation of their unsaturated components. Some of the remaining double bonds are isomerized (to trans) in this operation. These saturated and trans-fatty acid glycerides in the diet have been linked to long-term health issues such as atherosclerosis.

Triglycerides having three identical acyl chains, such as tristearin and triolein (above), are called "simple", while those composed of different acyl chains are called "mixed". If the acyl chains at the end hydroxyl groups (1 & 3) of glycerol are different, the center carbon becomes a chiral center and enantiomeric configurations must be recognized.
| The hydrogenation of vegetable oils to produce semisolid products has had unintended consequences. Although the hydrogenation imparts desirable features such as spreadability, texture, "mouth feel," and increased shelf life to naturally liquid vegetable oils, it introduces some serious health problems. These occur when the cis-double bonds in the fatty acid chains are not completely saturated in the hydrogenation process. The catalysts used to effect the addition of hydrogen isomerize the remaining double bonds to their trans configuration. These unnatural trans-fats appear to to be associated with increased heart disease, cancer, diabetes and obesity, as well as immune response and reproductive problems. |
Contributors
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry