7.1: Introduction to Alkyl Halides
- Page ID
- 30381
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Alkyl halides are also known as haloalkanes. This page explains what they are and discusses their physical properties. alkyl halides are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine). We will only look at compounds containing one halogen atom. For example:
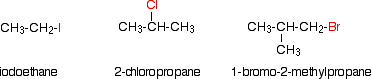
alkyl halides fall into different classes depending on how the halogen atom is positioned on the chain of carbon atoms. There are some chemical differences between the various types.
Primary alkyl halides
In a primary (1°) halogenoalkane, the carbon which carries the halogen atom is only attached to one other alkyl group.Some examples of primary alkyl halides include:
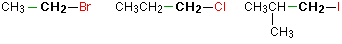
Notice that it doesn't matter how complicated the attached alkyl group is. In each case there is only one linkage to an alkyl group from the CH2 group holding the halogen. There is an exception to this: CH3Br and the other methyl halides are often counted as primary alkyl halides even though there are no alkyl groups attached to the carbon with the halogen on it.
Secondary alkyl halides
In a secondary (2°) halogenoalkane, the carbon with the halogen attached is joined directly to two other alkyl groups, which may be the same or different. Examples:
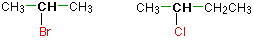
Tertiary alkyl halides
In a tertiary (3°) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different. Examples:
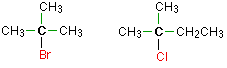
Contributors
Jim Clark (Chemguide.co.uk)

