21.3: Amines React as Bases and as Nucleophiles
- Page ID
- 14002
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Electrophilic Substitution at Nitrogen
Ammonia and many amines are not only bases in the Brønsted sense, they are also nucleophiles that bond to and form products with a variety of electrophiles. A general equation for such electrophilic substitution of nitrogen is:
2 R2ÑH + E(+)  R2NHE(+)
R2NHE(+)  R2ÑE + H(+) (bonded to a base)
R2ÑE + H(+) (bonded to a base)
A list of some electrophiles that are known to react with amines is shown here. In each case the electrophilic atom or site is colored red.
| Electrophile | RCH2–X | RCH2–OSO2R | R2C=O | R(C=O)X | RSO2–Cl | HO–N=O |
|---|---|---|---|---|---|---|
| Name | Alkyl Halide | Alkyl Sulfonate | Aldehyde or Ketone | Acid Halide or Anhydride | Sulfonyl Chloride | Nitrous Acid |
Alkylation
It is instructive to examine these nitrogen substitution reactions, using the common alkyl halide class of electrophiles. Thus, reaction of a primary alkyl bromide with a large excess of ammonia yields the corresponding 1º-amine, presumably by an SN2 mechanism. The hydrogen bromide produced in the reaction combines with some of the excess ammonia, giving ammonium bromide as a by-product. Water does not normally react with 1º-alkyl halides to give alcohols, so the enhanced nucleophilicity of nitrogen relative to oxygen is clearly demonstrated.
\[2 RCH_2Br + \underbrace{NH_3}_{\text{large excess}} \rightarrow RCH_2NH_2 + NH_4^{(+)} Br^{(–)}\]
It follows that simple amines should also be more nucleophilic than their alcohol or ether equivalents. If, for example, we wish to carry out an SN2 reaction of an alcohol with an alkyl halide to produce an ether (the Williamson synthesis), it is necessary to convert the weakly nucleophilic alcohol to its more nucleophilic conjugate base for the reaction to occur. In contrast, amines react with alkyl halides directly to give N-alkylated products. Since this reaction produces HBr as a co-product, hydrobromide salts of the alkylated amine or unreacted starting amine (in equilibrium) will also be formed.
2 RNH2 + C2H5Br  RNHC2H5 + RNH3(+) Br(–)
RNHC2H5 + RNH3(+) Br(–)  RNH2C2H5(+) Br(–) + RNH2
RNH2C2H5(+) Br(–) + RNH2
Unfortunately, the direct alkylation of 1º or 2º-amines to give a more substituted product does not proceed cleanly. If a 1:1 ratio of amine to alkyl halide is used, only 50% of the amine will react because the remaining amine will be tied up as an ammonium halide salt (remember that one equivalent of the strong acid HX is produced). If a 2:1 ratio of amine to alkylating agent is used, as in the above equation, the HX issue is solved, but another problem arises. Both the starting amine and the product amine are nucleophiles. Consequently, once the reaction has started, the product amine competes with the starting material in the later stages of alkylation, and some higher alkylated products are also formed. Even 3º-amines may be alkylated to form quaternary (4º) ammonium salts. When tetraalkyl ammonium salts are desired, as shown in the following example, Hünig's base may be used to scavenge the HI produced in the three SN2 reactions. Steric hindrance prevents this 3º-amine (Hünig's base) from being methylated.
C6H5NH2 + 3 CH3I + Hünig's base  C6H5N(CH3)3(+) I(–) + HI salt of Hünig's base
C6H5N(CH3)3(+) I(–) + HI salt of Hünig's base
The Hinsberg Test: Reaction with benzenesulfonyl chloride
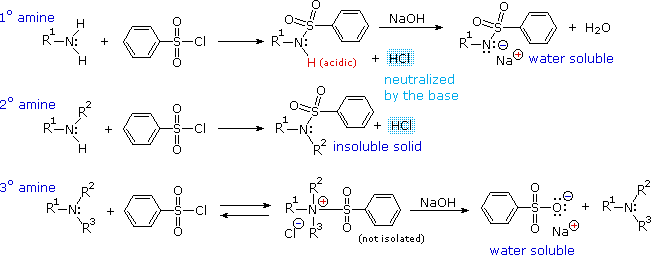
Another electrophilic reagent, benzenesulfonyl chloride, reacts with amines in a fashion that provides a useful test for distinguishing primary, secondary and tertiary amines (the Hinsberg test). As shown in the following equations, 1º and 2º-amines react to give sulfonamide derivatives with loss of HCl, whereas 3º-amines do not give any isolable products other than the starting amine. In the latter case a quaternary "onium" salt may be formed as an intermediate, but this rapidly breaks down in water to liberate the original 3º-amine (lower right equation).
The Hinsberg test is conducted in aqueous base (NaOH or KOH), and the benzenesulfonyl chloride reagent is present as an insoluble oil. Because of the heterogeneous nature of this system, the rate at which the sulfonyl chloride reagent is hydrolyzed to its sulfonate salt in the absence of amines is relatively slow. The amine dissolves in the reagent phase, and immediately reacts (if it is 1º or 2º), with the resulting HCl being neutralized by the base. The sulfonamide derivative from 2º-amines is usually an insoluble solid. However, the sulfonamide derivative from 1º-amines is acidic and dissolves in the aqueous base. Acidification of this solution then precipitates the sulfonamide of the 1º-amine.