3.12: Amines - Classification and Nomenclature
- Page ID
- 111234
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)learning objectives
- classify amines as primary, secondary, tertiary, quaternary, or heterocyclic
- name amines using IUPAC (systematic) and selected common name nomenclature
- draw the structure of amines from IUPAC (systematic) and selected common names
Amine bases are classified according to the number of alkyl or aryl groups attached to nitrogen. Amines are classified differently from alkyl halides and alcohols because nitrogen has a neutral bonding pattern of three bonds with a single lone pair. To classify amines, we look at the nitrogen atom of the amine and count the number of alkyl groups bonded to it. This number is the classification of the amine.
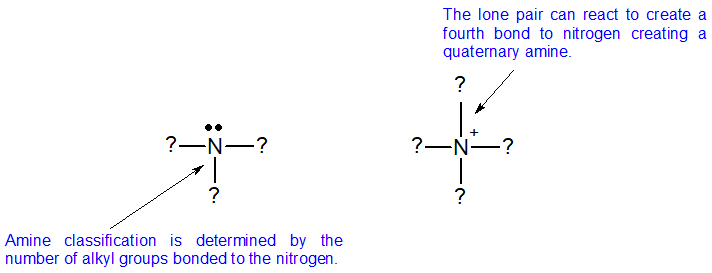

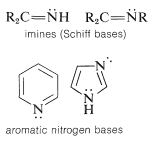
There are two additional classifications of amines. When the nitrogen is double bonded to carbon, then it is call am imine. When nitrogen is part of a ring that includes double bonds, then it is classified as heterocyclic, as seen in the aromatic nitrogen bases shown below.
Nomenclature
Amines are derivatives of ammonia in which one or more of the hydrogens has been replaced by an alkyl or aryl group. Amino compounds can be named either as derivatives of ammonia or as amino-substituted compounds:

The nomenclature of amines is further complicated by the fact that several different nomenclature systems exist, and there is no clear preference for one over the others. The four compounds shown in the top row of the following diagram are all C4H11N isomers. The first two are classified as 1º-amines, since only one alkyl group is bonded to the nitrogen; however, the alkyl group is primary in the first example and tertiary in the second. The third and fourth compounds in the row are 2º and 3º-amines respectively. The bottom row shows the structures for some common amines that need to be memorized.
- The Chemical Abstract Service has adopted a nomenclature system in which the suffix -amine is attached to the root alkyl name. For 1º-amines such as butanamine (first example) this is analogous to IUPAC alcohol nomenclature (-ol suffix). The additional nitrogen substituents in 2º and 3º-amines are designated by the prefix N- before the group name. These CA names are colored magenta in the diagram.
- Finally, a common system for simple amines names each alkyl substituent on nitrogen in alphabetical order, followed by the suffix -amine. These are the names given in the last row (colored black).
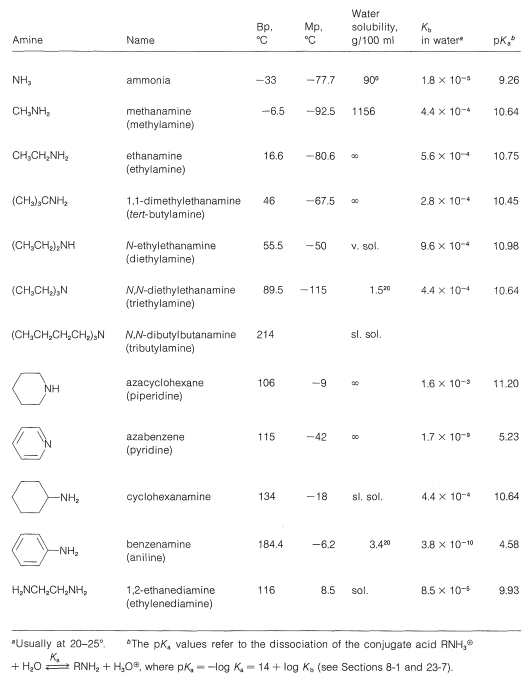
To be consistent and logical in naming amines as substituted ammonias, they strictly should be called alkanamines and arenamines, according to the nature of the hydrocarbon grouping. Unfortunately, the term alkylamine is used very commonly in place of alkanamine, while a host of trivial names are used for arenamines. We shall try to indicate both the trivial and the systematic names where possible. Some typical amines, their names, and their physical properties are listed in he Table below. The completely systematic names give in the Table illustrate the difficulty one gets into by using completely systematic names, and why simpler but less systematic names continue to be used for common compounds. A good example is \(\ce{N}\),\(\ce{N}\)-dibutylbutamine versus tributylamine. The special ways of naming heterocyclic amines can be referenced in the appendix of this chapter.
Common Amines and Their Properties
Alkaloids
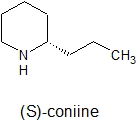
Many biologically important compounds are amines. Alkaloids are amines synthesized by plants to protect them from being eaten. Humans primarily use alkaloids medicinally as pain killers. All alkaloids are toxic and addictive. The Greeks killed Socrates with (S)-coniine. Mild cases of alkaloid poisoning produce psychological effects resembling peacefulness, euphoria or hallucinations.
Ammonium Salts
A nitrogen bonded to four alkyl groups will necessarily be positively charged, and is called a 4º-ammonium cation. For example, (CH3)4N(+) Br(–) is tetramethylammonium bromide. Salts of amines with inorganic or organic acids are named as substituted ammonium salts, except when the nitrogen is part of a ring system. Examples are
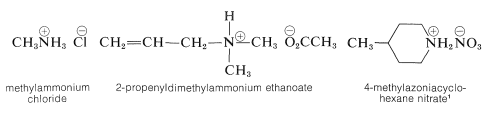
\(^1\)Note the use of azonia to denote the cationic nitrogen in the ring, whereas aza is used for neutral nitrogen.
Perhaps the most noteworthy aspect of ammonium salts is that they have low odor and are water soluble. These qualities are explored more fully in the amine chapter
Heterocyclic Amine Nomenclature
Heterocyclic amines are amines in which the nitrogen is part of a ring that contains at least one double bond. Many aromatic and heterocyclic amines are known by unique common names, the origins of which are often unknown to the chemists that use them frequently. Since these names are not based on a rational system, it is necessary to memorize them. There is a systematic nomenclature of heterocyclic compounds, but it will not be discussed here. For further details, refer to the appendix of this chapter for the full IUPAC rules of organic compound nomenclature.
Exercise
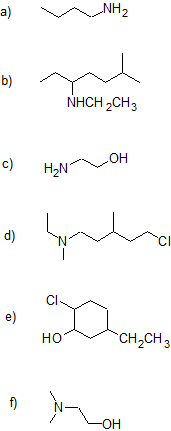
1. Draw the bond-line structure for each compound and write the condensed structural formula for parts (a) & (d) - (g).
a) 3-bromo-pentan-2-amine
b) cyclopentanamine
c) trans-3-ethylcyclohexanamine
d) sec-butyl tert-butyl amine
e) N,N-dimethyl-3-pentanamine
f) 4-methyl-2-hexanamine
g) 6-bromo-4-amino-2-heptanol
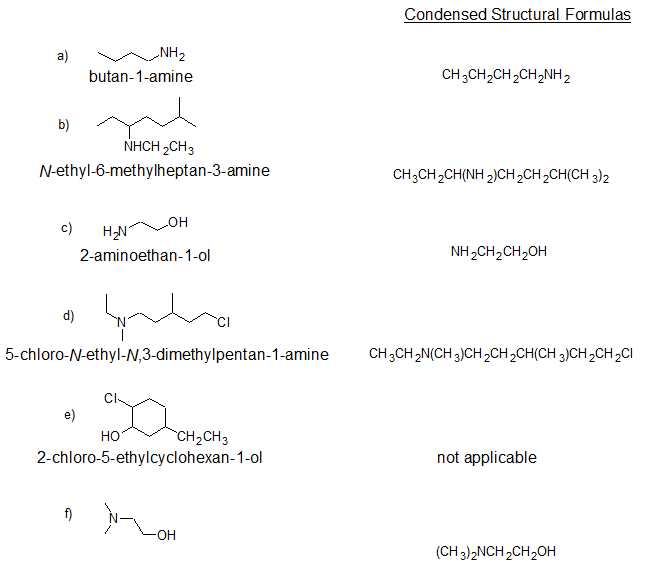
2. Give the IUPAC name and condensed structural formula for each compound.
- Answer
-
1.
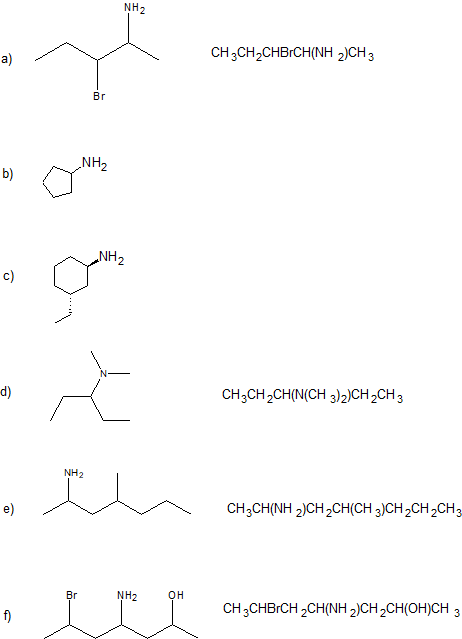
2.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."
Contributors


