11.5: Preparation of Alkynes
- Page ID
- 30477
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)lkynes can be a useful functional group to synthesize due to some of their antibacterial, antiparasitic, and antifungal properties. One simple method for alkyne synthesis is by double elimination from a dihaloalkane.
Introduction
One case in which elimination can occur is when a haloalkane is put in contact with a nucleophile. The table below is used to determine which situations will result in elimination and the formation of a π bond.
| Type of Haloalkane | Weak Base, Poor Nucleophile | Weak Base, Good Nucleophile | Strong, Unhindered Base | Strong, Hindered Base |
| Primary | ||||
| Unhindered | E2 | |||
| Branched | E2 | E2 | ||
| Secondary | E1 | E2 | E2 | |
| Tertiary | E1 | E1 | E2 | E2 |
* Empty Box means no elimination or Pi bond forms
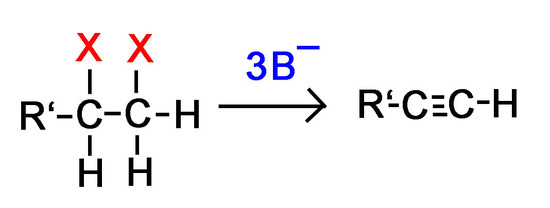
To synthesize alkynes from dihaloalkanes we use dehydrohalogenation. The majority of these reactions take place using alkoxide bases (other strong bases can also be used) with high temperatures. This combination results in the majority of the product being from the E2 mechanism.
E2 Mechanism
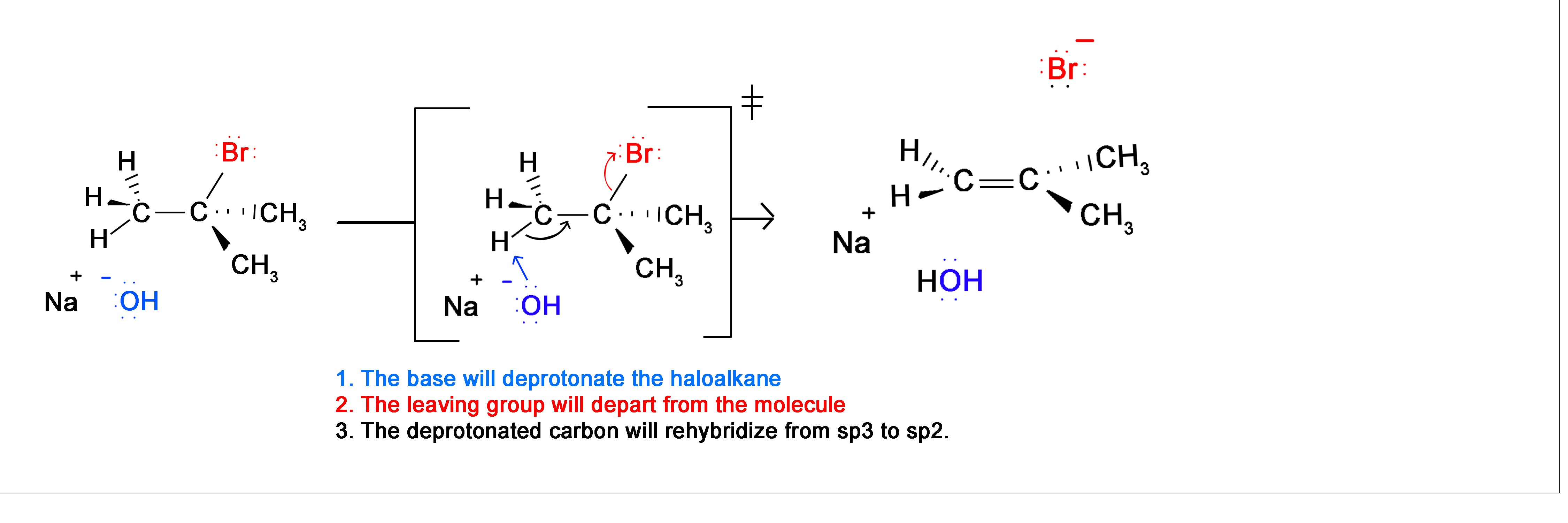
Recall that the E2 mechanism is a concerted reaction (occurs in 1 step). However, in this 1 step there are 3 different changes in the molecule. This is the reaction between 2-Bromo-2-methylpropane and Sodium Hyrdoxide.
This is a brief review of the E2 reaction. For further information on why the reaction proceeds as it does visit the E2 reaction page. Now, if we apply this concept using 2 halides on vicinal or geminal carbons, the E2 reaction will take place twice resulting in the formation of 2 Pi bonds and thus an Alkyne.
Dihaloalkane Elimination
This is a general picture of the reaction taking place without any of the mechanisms shown.
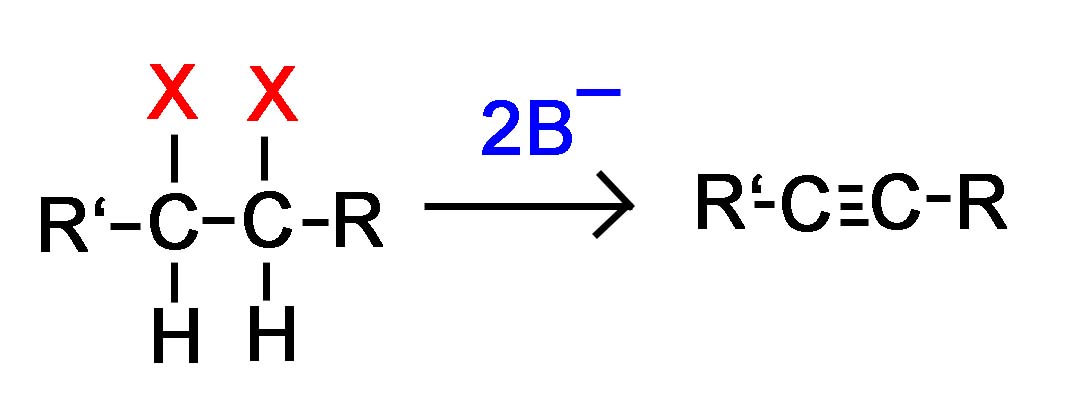
or
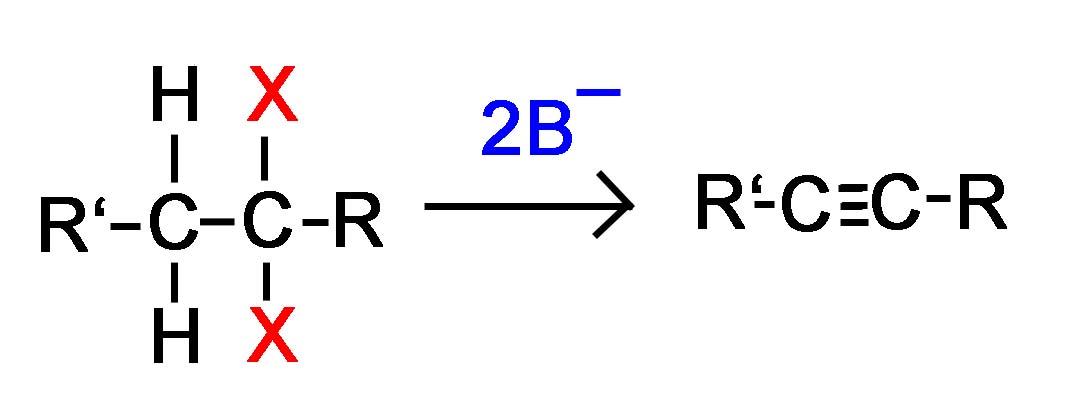
* With a terminal haloalkane the equation above is modified in that 3 equivalents of base will be used instead of 2.
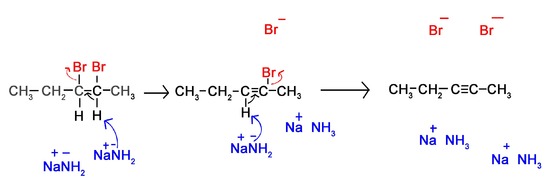
Lets look at the mechanism of a reaction between 2,3-Dibromopentane with sodium amide in liquid ammonia.
- Liquid ammonia is not part of the reaction, but is used as a solvent
- Notice the intermediate of the alkyne synthesis. It is stereospecifically in its anti form. Because the second proton and halogen are pulled off the molecule this is unimportant to the synthesis of alkynes. For more information on this see the page on preparation of alkenes from haloalkanes.
Preparation of Alkynes from Alkenes
Lastly, we will briefly look at how to prepare alkynes from alkenes. This is a simple process using first halogenation of the alkene bond to form the dihaloalkane, and next, using the double elimination process to protonate the alkane and from the 2 Pi bonds.
This first process is gone over in much greater detail in the page on halogenation of an alkene. In general, chlorine or bromine is used with an inert halogenated solvent like chloromethane to create a vicinal dihalide from an alkene. The vicinal dihalide formed is the reactant needed to produce the alkyene using double elimination, as covered previously on this page.

In The Lab
Due to the strong base and high temperatures needed for this reaction to take place, the triple bond may change positions. An example of this is when reactants that should form a terminal alkyne, form a 2-Alkyne instead. The use of NaNH2 in liquid NH3 is used in order to prevent this from happening due to its lower reacting temperature. Even so, most chemists will prefer to use nucleophilic substitution instead of elimination when trying to form a terminal alkyne.
Questions
Question 1: Why would we need 3 bases for every terminal dihaloalkane instead of 2 in order to form an alkyne?
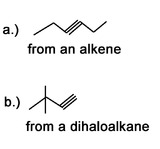
Question 2: What are the major products of the following reactions:
a.) 1,2-Dibromopentane with sodium amide in liquid ammonia
b.) 1-Pentene first with Br2 and chloromethane, followed by sodium ethoxide (Na+ -O-CH2CH3)
Question 3: What would be good starting molecules for the synthesis of the following molecules:
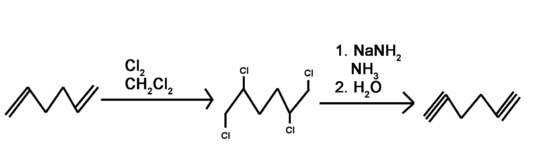
Question 4: Use a 6 carbon diene to synthesize a 6 carbon molecule with 2 terminal alkynes.
Answers
Answer 1: Remember that hydrogen atoms on terminal alkynes make the alkyne acidic. One of the base molecules will pull off the terminal hydrogen instead of one of the halides like we want.
Answer 2:
a.) 1-Pentyne
b.) 1-Pentyne
Answer 3:
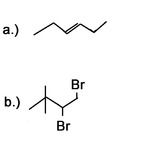
Answer 4: Bromine or chlorine can be used with different inert solvents for the halogenation. This can be done using many different bases. Liquid ammonia is used as a solvent and needs to be followed by an aqueous work-up.
Outside links
References
- Vollhardt, Peter, and Neil Shore. Organic Chemistry: Structure and Function. 5th. New York: W.H. Freeman and Company, 2007.
- Daley, Richard, and Sally Daley. "13.8 Elimination of Organohalogens." Organic Chemistry. Daley. 5 July 2005. 21 Feb. 2009. <http://www.ochem4free.info/node/143>.