16.9: Halogenation
- Page ID
- 30769
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Halogenation is an example of electrophillic aromatic substitution. In electrophilic aromatic substitutions, a benzene is attacked by an electrophile which results in substition of hydrogens. However, halogens are not electrophillic enough to break the aromaticity of benzenes, which require a catalyst to activate.
Activation of Halogen
(where X= Br or Cl, we will discuss further in detail later why other members of the halogen family Flourine and Iodine are not used in halogenation of benzenes)
.jpg?revision=1&size=bestfit&width=272&height=170)
Hence, Halogen needs the help and aid of Lewis Acidic Catalysts to activate it to become a very strong electrophile. Examples of these activated halogens are Ferric Hallides (FeX3) Aluminum Halides (AlX3) where X= Br or Cl. In the following examples, the halogen we will look at is Bromine.
In the example of bromine, in order to make bromine electrophillic enough to react with benzene, we use the aid of an aluminum halide such as aluminum bromide.
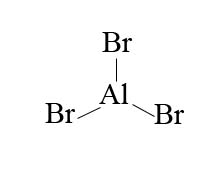
With aluminum bromide as a Lewis acid, we can mix Br2 with AlBr3 to give us Br+. The presence of Br+ is a much better electrophile than Br2 alone. Bromination is acheived with the help of AlBr3 (Lewis acid catalysts) as it polarizes the Br-Br bond. The polarization causes polarization causes the bromine atoms within the Br-Br bond to become more electrophillic. The presence of Br+ compared to Br2 alone is a much better electrophille that can then react with benzene.
.jpg?revision=1&size=bestfit&width=639&height=214)
As the bromine has now become more electrophillic after activation of a catalyst, an electrophillic attack by the benzene occurs at the terminal bromine of Br-Br-AlBr3. This allows the other bromine atom to leave with the AlBr3 as a good leaving group, AlBr4-.
.jpg?revision=1&size=bestfit&width=406&height=203)
.jpg?revision=1&size=bestfit&width=482&height=283)
After the electrophilic attack of bromide to the benzene, the hydrogen on the same carbon as bromine substitutes the carbocation in which resulted from the attack. Hence it being an electrophilic aromatic SUBSTITUTION. Since the by-product aluminum tetrabromide is a strong nucleophile, it pulls of a proton from the Hydrogen on the same carbon as bromine.
.jpg?revision=1&size=bestfit&width=481&height=224)
In the end, AlBr3was not consumed by the reaction and is regenerated. It serves as our catalyst in the halogenation of benzenes.
Dissociation Energies of Halogens and its Effect on Halogenation of Benzenes
The electrophillic bromination of benzenes is an exothermic reaction. Considering the exothermic rates of aromatic halogenation decreasing down the periodic table in the Halogen family, Flourination is the most exothermic and Iodination would be the least. Being so exothermic, a reaction of flourine with benzene is explosive! For iodine, electrophillic iodination is generally endothermic, hence a reaction is often not possible. Similar to bromide, chlorination would require the aid of an activating presence such as Alumnium Chloride or Ferric Chloride. The mechanism of this reaction is the same as with Bromination of benzene.
References
- Vollhardt, Peter, and Neil Shore. Organic Chemistry: Structure and Function. 5th Edition. New York: W.H. Freeman and Company, 2007.
Problems
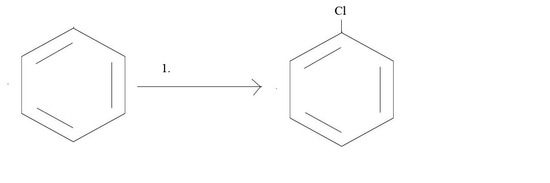
1. What reagents would you need to get the given product?
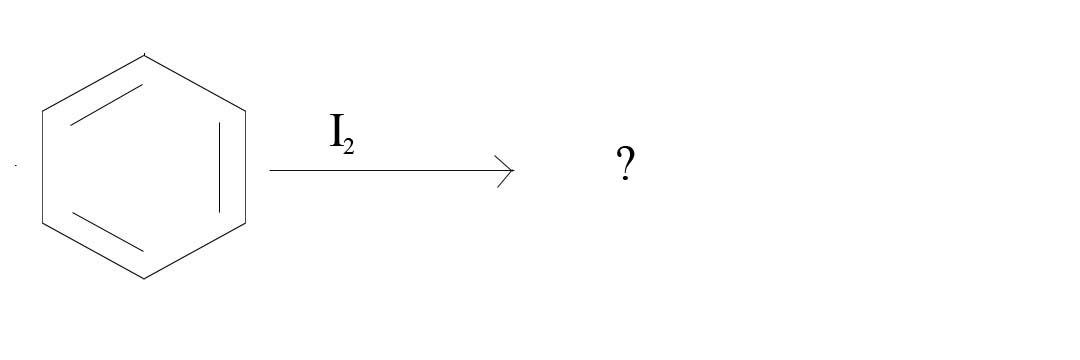
2. What product would result from the given reagents?
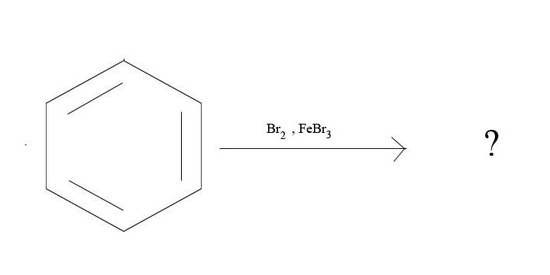
3. What is the major product given the reagents below?
4. Draw the formatin of Cl+ from AlCl3 and Cl2
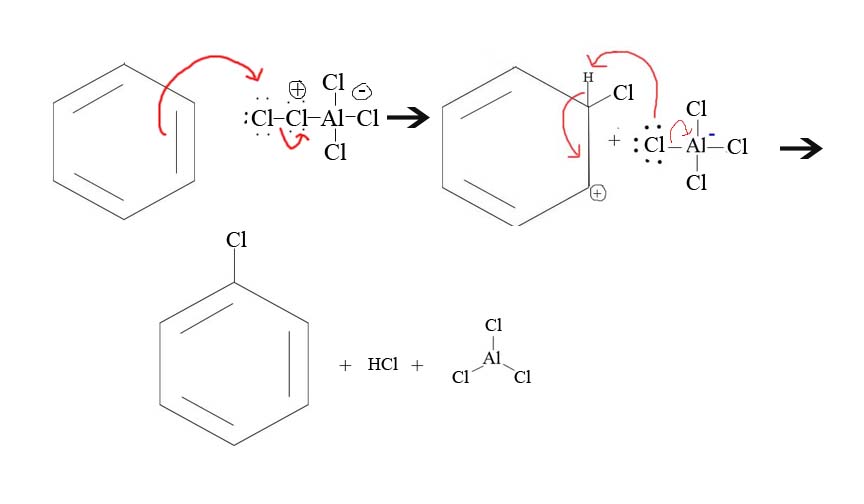
5. Draw the mechanism of the reaction between Cl+ and a benzene.
Solutions
1. Cl2 and AlCl3 or Cl2 and FeCl3
2. No Reaction
3.
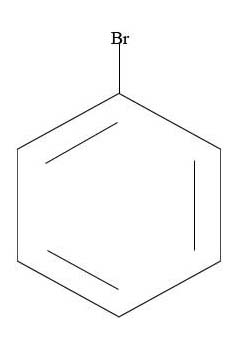
4.
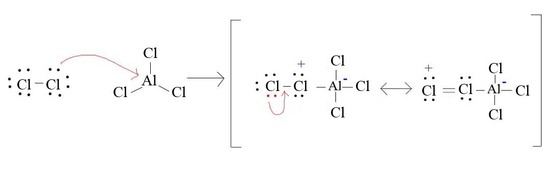
5.
Contributors
- Catherine Nguyen

