2.7 Mass Spectrometry of Some Common Functional Groups
- Page ID
- 432165
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Learn common fragmentation patterns for functional groups
- Interpret the fragmentations of mass spectra
Some fragment ions are very common in mass spectrometry. These ions are seen frequently for either of two reasons:
- there is not a pathway available to break the ion down.
- the ion is relatively stable, so it forms easily.
There are a number of ions commonly seen in mass spectrometry that tell you a little bit about the structure. Just like with anions, there are a couple of common factors influence cation stability:
- Electronegativity plays a role. More electronegative atoms are less likely to be cations.
- Polarizability also plays a role. More polarizable atoms are more likely to be cations.
- Delocalization stabilizes a cation by spreading out the charge onto two or more different atoms. Resonance is a common way to delocalize charge.
Alcohols
When alcohols are subjected to ionization, two fragmentation pathways can occur - alpha cleavage and dehydration. Alpha cleavage occurs by a C-C bond nearest the hydroxyl group being broken. This yields a neutral radical plus a resonance stabilized, oxygen-containing cation. As an example let's look at the mass spectrum of 2-pentanol below.
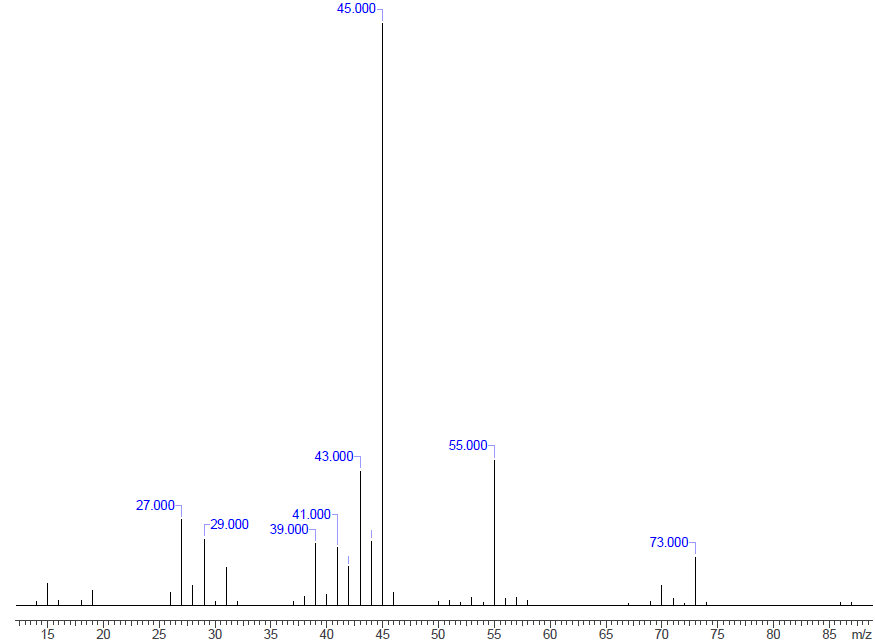
The parent peak is m/z = 88. The base peak is m/z = 45, which correlates to a fragmentation that occurs with an alpha cleavage. This fragment is the piece due to the resonance stabilized oxygen-containing cation, which is shown in the pathway below.
In dehydration, water is eliminated. This leaves an alkene radical cation that is 18 units less than the molecular ion peak. Using our example of 2-pentanol, this would lead to a peak at m/z = 70 and there is.
Amines
A general principle when nitrogen is part of a molecule is that if there is an odd number of nitrogens, then the molecular weight will be an odd number. This is also known as the nitrogen rule and stems from the fact that nitrogen is a trivalent atom. It also goes that if the molecule contains an even molecular weight, then there will be zero or two nitrogen atoms.
As with alcohols, primary amines undergo a characteristic alpha cleavage. As an example, the mass spectrum of 2-aminopentane is below.
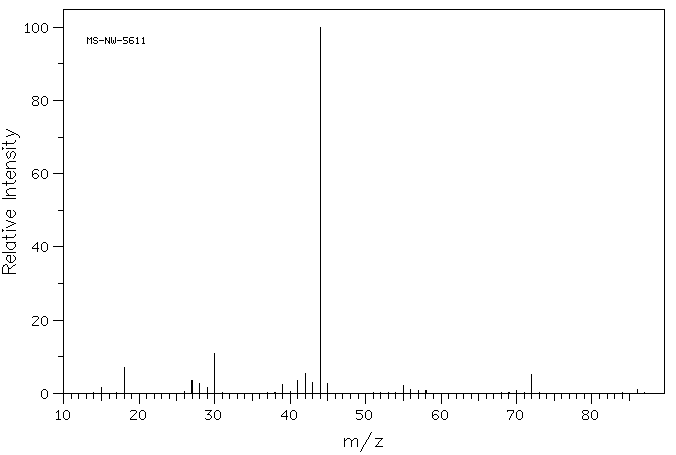
Source: SDBSWeb : https://sdbs.db.aist.go.jp/sdbs/cgi-..._frame_top.cgi (National Institute of Advanced Industrial Science and Technology of Japan) [Accessed August 16, 2022]
The parent peak is very small, but is at m/z = 87. The base peak (m/z = 44) is due to an alpha cleavage and forming a fragment of a resonance stabilized nitrogen-containing cation (see pathway below).
Halides
Halides have isotopes that give distinct appearances in a mass spectrum. Chlorine has two isotopes 35C and 37C with a 3:1 ratio (roughly). This ratio shows up in the mass spectrum for a chlorine-containing compound. Below is the mass spectrum of 2-chloro-2-methylpropane.
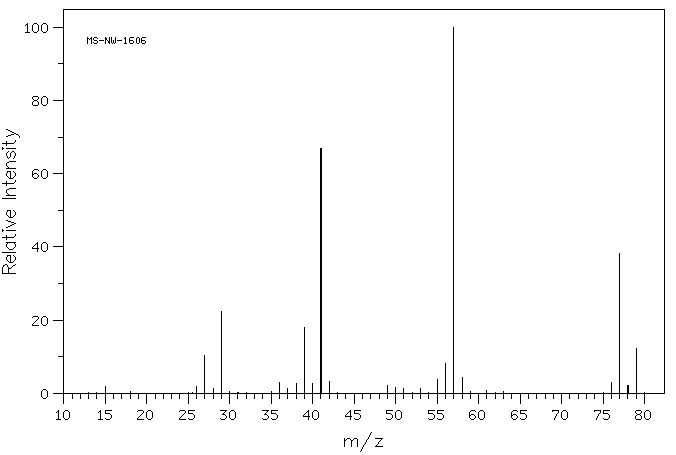
Source: SDBSWeb : https://sdbs.db.aist.go.jp/sdbs/cgi-..._frame_top.cgi (National Institute of Advanced Industrial Science and Technology of Japan) [Accessed August 16, 2022]
Looking at the molecular ion peak (m/z = 77 peak), there is another peak at m/z= 79. The peak at 79 is called the M + 2 peak. The ratio of the relative abundance/intensity of the M:M + 2 is about 3:1, which reflecting the isotopic abundance of 35C:37C.
With bromine, the isotopic distribution of 79Br and 81Br is more like 50:50. Again, the ratio of the relative abundance/intensity of the M:M + 2 is about 50:50. In the example below, the mass spectrum of 1-bromohexane is shown.
Source: SDBSWeb : https://sdbs.db.aist.go.jp/sdbs/cgi-..._frame_top.cgi (National Institute of Advanced Industrial Science and Technology of Japan) [Accessed August 16, 2022]
The roughly 50:50 distribution can be seen in the parent peak and M + 2 (m/z = 164 and 165). It is again showing up at the peaks m/z = 135 and 137. The two peaks in each are nearly the same height. In the fragments that contain the bromine, this ratio will be reflected.
Carbonyl Compounds
The McLafferty rearrangement is a common cleavage that occurs in carbonyl compounds that have a hydrogen three atoms away from carbonyl group. This rearragnement yields a carbonyl-containing radical cation via β-cleavage to produce an enol cation and an alkene. This fragmentation is shown below.
In the mass spectrum of 2-hexanone (below), the m/z peak = 59 represents the enol cation that would form from the McLafferty rearrangement.
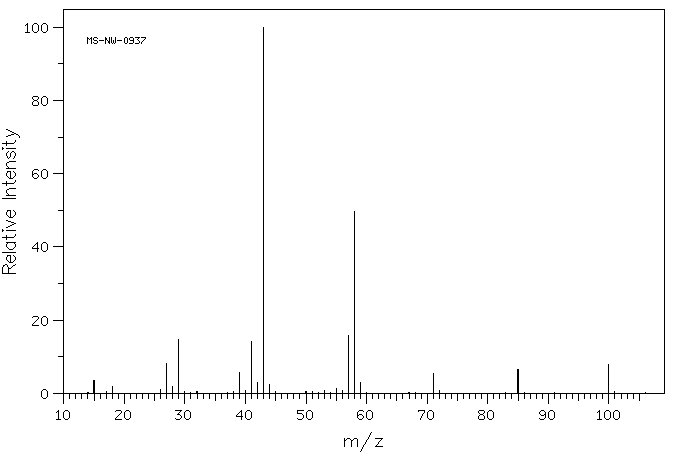
Source: SDBSWeb : https://sdbs.db.aist.go.jp/sdbs/cgi-..._frame_top.cgi (National Institute of Advanced Industrial Science and Technology of Japan) [Accessed August 16, 2022]
The carbonyl compounds can also undergo alpha cleavage as was seen with the alcohol and primary amines. The alpha cleavage occurs between the carbonyl carbon and the neighboring carbon yielding an acylium ion and a neutral radical (see below).
The acylium ion has an m/z =43. This fragment shows up at m/z = 43. This cleavage can also be seen as the loss of the acylium ion from the parent ion (100-43 = 57). There is a m/z peak at 57 to represent this loss.
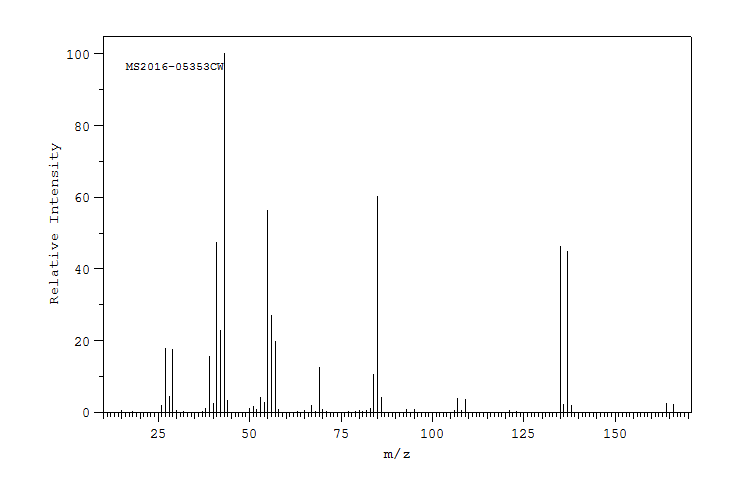
The mass spectrum of 2-methyl-3-penatonol is shown below.
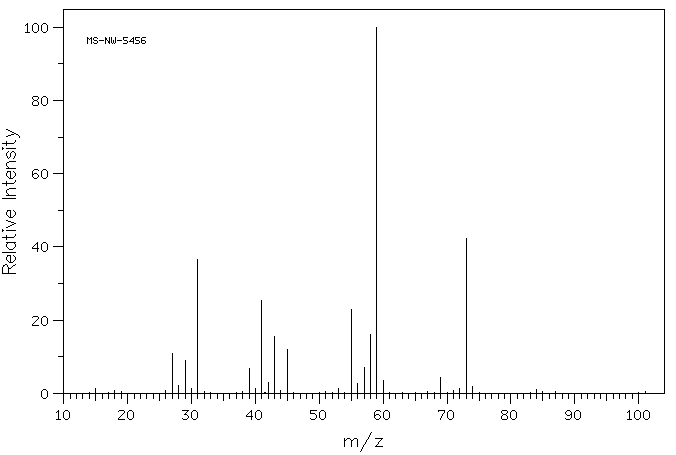
Source: SDBSWeb : https://sdbs.db.aist.go.jp/sdbs/cgi-...p;sdbsno=13488 (National Institute of Advanced Industrial Science and Technology of Japan) [Accessed August 16, 2022]
What fragments can you identify?
Solution
First, you want to look up the draw or look up the structure. 2-methyl-3-pentanol:
Next, calculate the mass of the molecular ion and identify the functional groups in the molecule. M+1 = 102 and there is an alcohol present.
Then write down the fragmentation patterns you may expect and calculate the masses for those peaks and compare it to the mass spectrum. With an alcohol, there are two pathways for fragmentation - alpha cleavage and dehydration. From the alpha cleavage, one would expect two peaks m/z = 73 and 59. From dehydration, one would expect an m/z of 84. The dehydration fragment peak is not observed, but both the fragments from the alpha cleavage are observed.
What are the masses of the charged fragments produced in the following cleavage pathways?
a. alpha cleavage of triethylamine
b. McLafferty rearrangment of 4-methyl-2-pentanone
- Answer
-
a. m/z = 86
b. m/z = 58
Nicotine is a diamino compound with two rings and a molecular ion peak of 162.1157. Remembering the nitrogen rule, give the molecular formula for nicotine and calculate the number of double bonds.
- Answer
-
C10H14N2

