1.2: The Nature of Radiant Energy and Electromagnetic Radiation
- Page ID
- 432152
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
After reading this section, you should be able to
- Understand the nature of light
- Explain what it meant by wave and particle duality
- Write a brief paragraph discussing the nature of electromagnetic radiation.
- Explain the equations that relate energy to frequency, frequency to wavelength and energy to wavelength, and perform calculations using these relationships.
Make certain that you can define, and use in context, the key terms below.
- electromagnetic radiation
- electromagnetic spectrum
- hertz (Hz)
- photon
- wavelength
- constructive interference
- destructive interference
As you read the print off this computer screen now, you are reading pages of fluctuating energy and magnetic fields. Light, electricity, and magnetism are all different forms of electromagnetic radiation. Electromagnetic radiation, as you may recall from a previous chemistry or physics class, is composed of electrical and magnetic waves which oscillate on perpendicular planes as shown in the diagram below. These electric and magnetic waves travel at 90 degree angles to each other and have certain characteristics, including amplitude, wavelength, and frequency. Electron radiation is released as photons, which are bundles of light energy that travel at the speed of light as quantized harmonic waves. This radiation can travel through empty space. Most other types of waves must travel through some sort of substance. For example, sound waves need either a gas, solid, or liquid to pass through in order to be heard.This energy is then grouped into categories based on its wavelength into the electromagnetic spectrum.
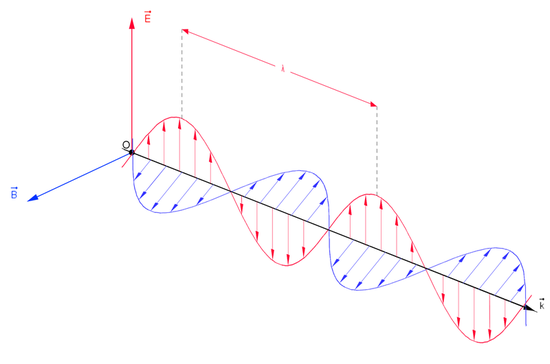
Waves and their Characteristics
Just like ocean waves, electromagnetic waves travel in a defined direction. While the speed of ocean waves can vary, however, the speed of electromagnetic waves – commonly referred to as the speed of light (2.99 x 108 m/s) – is essentially a constant, approximately 300 million meters per second. This is true whether we are talking about gamma radiation or visible light. Obviously, there is a big difference between these two types of waves – we are surrounded by the latter for more than half of our time on earth, whereas we hopefully never become exposed to the former to any significant degree.
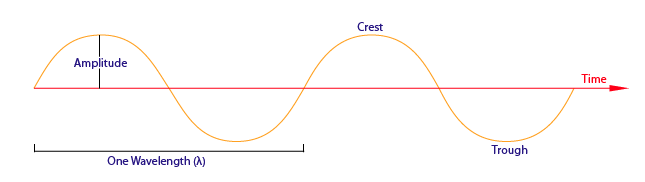
Amplitude
Amplitude as shown above is the distance from the maximum vertical displacement of the wave to the middle of the wave. This measures the magnitude of oscillation of a particular wave. In short, the amplitude is basically the height of the wave. Larger amplitude means higher energy and lower amplitude means lower energy. Amplitude is important because it tells you the intensity or brightness of a wave in comparison with other waves.
Wavelength
The different properties of the various types of electromagnetic radiation are due to differences in their wavelengths, and the corresponding differences in their energies: shorter wavelengths correspond to higher energy. Wavelength (\(\lambda\)) is the distance of one full cycle of the oscillation (measured between the distance of either crest to crest as shown above or trough to trough). Longer wavelength waves such as radio waves carry low energy; this is why we can listen to the radio without any harmful consequences. Shorter wavelength waves such as x-rays carry higher energy that can be hazardous to our health. Consequently lead aprons are worn to protect our bodies from harmful radiation when we undergo x-rays. This wavelength frequently relationship is characterized by:
\[ c = \lambda\nu \]
where
- c is the speed of light,
- \( \lambda \) is wavelength, and
- \( \nu \) is frequency.
Shorter wavelength means greater frequency, and greater frequency means higher energy. Wavelengths are important in that they tell one what type of wave one is dealing with. You can see this depicted in the image below.
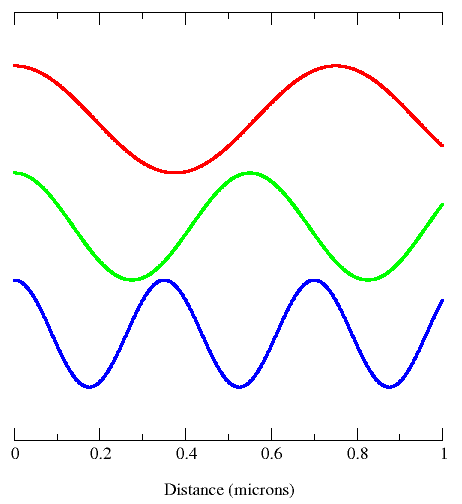
Remember, wavelength tells you the type of light and amplitude tells you about the intensity of the light.
Frequency
Frequency is defined as the number of cycles per second, and is expressed as sec-1 or Hertz (Hz). Frequency is directly proportional to energy and can be express as:
\[ E = h\nu \]
where
- E is energy,
- h is Planck's constant, (h= 6.62607 x 10-34 J), and
- \(\nu\) is frequency.
Period
Period (T) is the amount of time a wave takes to travel one wavelength; it is measured in seconds (s).
Velocity
The velocity of wave in general is expressed as:
\[ velocity = \lambda\nu \]
Remember for an electromagnetic wave, the velocity in vacuum is \(2.99 \times 10^8\;m/s\) or \(186,282\) miles/second.
Electromagnetic spectrum
The notion that electromagnetic radiation contains a quantifiable amount of energy can perhaps be better understood if we talk about light as a stream of particles, called photons, rather than as a wave. (Recall the concept known as ‘wave-particle duality’: at the quantum level, wave behavior and particle behavior become indistinguishable, and very small particles have an observable ‘wavelength’). If we describe light as a stream of photons, the energy of a particular wavelength can be expressed as:
\[E = \dfrac{hc}{\lambda} \tag{12.5.1}\]
where E is energy in J, λ (the Greek letter lambda) is wavelength in meters, c is 3.00 x 108 m/s (the speed of light), and h is 6.626 × 10−34 J · s, a number known as Planck’s constant.
Because electromagnetic radiation travels at a constant speed, each wavelength corresponds to a given frequency, which is the number of times per second that a crest passes a given point. Longer waves have lower frequencies, and shorter waves have higher frequencies. Frequency is commonly reported in hertz (Hz), meaning ‘cycles per second’, or ‘waves per second’. The standard unit for frequency is s-1.
When talking about electromagnetic waves, we can refer either to wavelength or to frequency - the two values are interconverted using the simple expression:
\[ \lambda \nu = c \tag{12.5.2}\]
where ν (the Greek letter ‘nu’) is frequency in s-1. Visible red light with a wavelength of 700 nm, for example, has a frequency of 4.29 x 1014 Hz, and an energy of 2.84 x 10-19 J per photon or 171 kJ per mole of photons (remember Avogadro’s number = 6.02 × 1023 mol−1). The full range of electromagnetic radiation wavelengths is referred to as the electromagnetic spectrum (below).
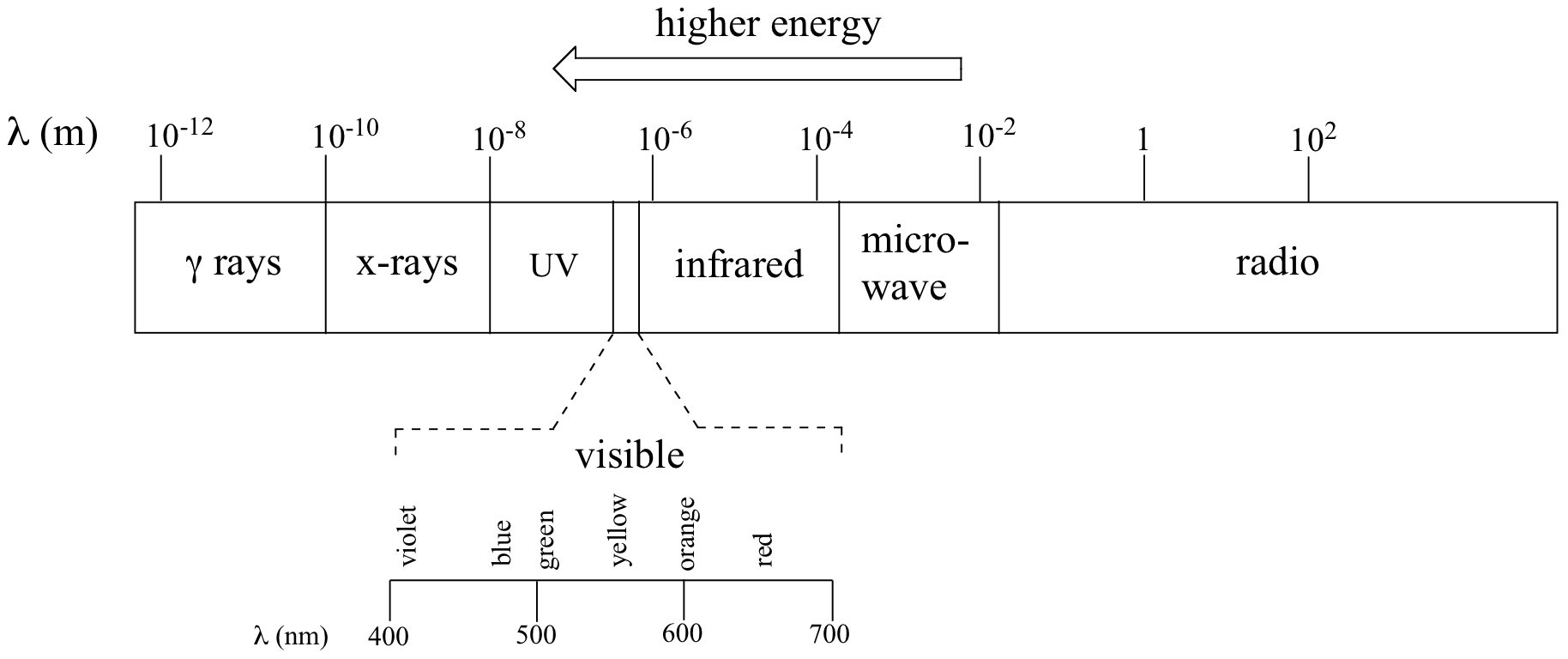
Visible light has a wavelength range of about 400-700 nm. What is the corresponding frequency range? What is the corresponding energy range, in kJ mol−1 of photons?
Solution
For light with a wavelength of 400 nm, the frequency is 7.50 × 1014 Hz:
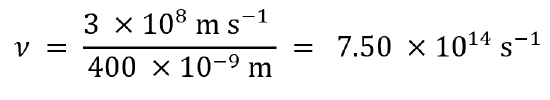
In the same way, we calculate that light with a wavelength of 700 nm has a frequency of 4.29 × 1014 Hz.
To calculate corresponding energies using hc/λ. We find for light at 400 nm:
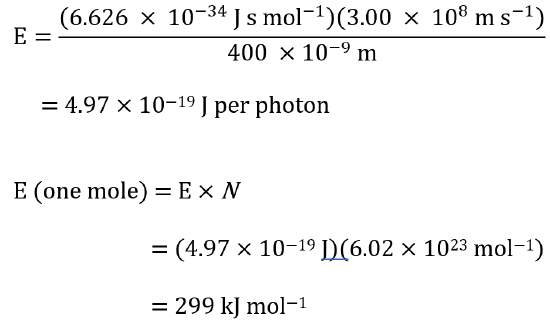
Using the same equation, we find that light at 700 nm corresponds to 171 kJ mol−1.
As a wave’s wavelength increases, the frequency decreases, and as wave’s wavelength decreases, the frequency increases. When electromagnetic energy is released as the energy level increases, the wavelength decreases and frequency decreases. Thus, electromagnetic radiation is then grouped into categories based on its wavelength or frequency into the electromagnetic spectrum. The different types of electromagnetic radiation shown in the electromagnetic spectrum consists of radio waves, microwaves, infrared waves, visible light, ultraviolet radiation, X-rays, and gamma rays. The part of the electromagnetic spectrum that we are able to see is the visible light spectrum.
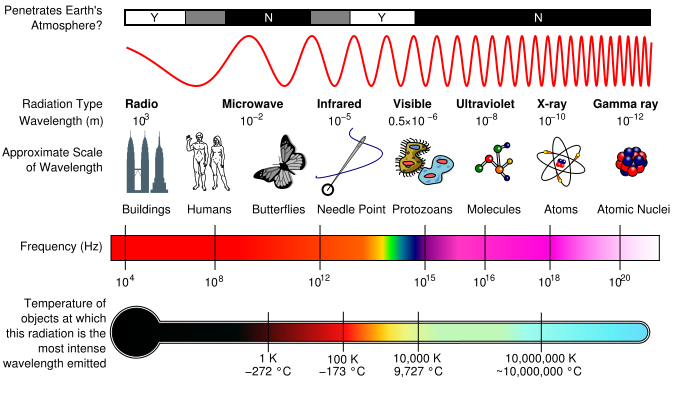
Electromagnetic Spectrum with Radiation Types
How are the types of radiation related to me? Radio waves are transmitted by radio broadcasts, TV broadcasts, and even cell phones. They are also used in radar systems, where they release radio energy and collect the bounced energy back. Microwaves can be used to broadcast information through space, as well as warm food. Infrared radiation can be released as heat or thermal energy and is most commonly used in remote sensing as infrared sensors collect thermal energy, providing us with weather conditions. Visible light is the only part of the electromagnetic spectrum that humans can see with an unaided eye. This part of the spectrum includes a range of different colors that all represent a particular wavelength. Rainbows are formed in this way; light passes through matter in which it is absorbed or reflected based on its wavelength. Thus, some colors are reflected more than other, leading to the creation of a rainbow. The typical wavelengths of each color region is listed below.
|
Color Region |
Wavelength (nm) |
| Violet | 380-435 |
| Blue | 435-500 |
| Cyan | 500-520 |
| Green | 520-565 |
| Yellow | 565-590 |
| Orange | 590-625 |
| Red | 625-740 |
Ultraviolet, Radiation, X-Rays, and Gamma Rays are all related to events occurring in space. UV radiation is most commonly known because of its severe effects on the skin from the sun, leading to cancer. X-rays are used to produce medical images of the body. Gamma Rays can used in chemotherapy in order to rid of tumors in a body since it has such a high energy level. Out this huge spectrum, the human eyes can only detect waves from 390 nm to 780 nm.
Calculate the energies for the following;
A. Gamma Ray λ = 4.0x10-11 m
B. X-Ray λ = 4.0x10-9 m
C. UV light υ = 5.0x1015 Hz
D. Infrared Radiation λ = 3.0x10-5 m
E. Microwave Radiation υ = 3.0x1011 Hz
- Answer
-
A. 4.965x10-15 J
B. 4.965x10-17 J
C. 3.31x10-18 J
D. 6.62x10-21 J
E. 1.99x10-22
Note: You should not try to memorize the relationship between energy and wavelength in the form in which it is given here. Instead, you should be prepared to work from first principles using:
E = hv, where h = Plank's constant = 6.626 × 10−34J · s.
c = λv, where c = the speed of light = 3.00 × 108m · s−1.
Avogadro’s number = 6.02 × 1023 mol−
What is the frequency of a wave with a wavelength of 200 cm?
- Answer
-
1.5 × 108 Hz
Which of the following frequencies/wavelengths are higher energy?
A. λ = 2.0x10-6 m or λ = 3.0x10-9 m
B. υ = 3.0x109 Hz or υ = 3.0x10-6 Hz
- Answer
-
A. λ = 3.0x10-9 m
B. υ = 3.0x109 H
A radio transmits a frequency of 100 Hz. What is the wavelength of this wave?
- Answer
-
2.998 × 106 m
References
- Atkins, Peter and Julio de Paula. Physical Chemistry for the Life Sciences. New York: Oxford University Press, 2006.
- Chang, Raymond. Physical Chemistry for the Biosciences. USA: University Science Books, 2005.
- McQuarrie, Donald and Simon, John. Physical Chemistry: A Molecular Approach. Sausalito, CA: University Science Books, 1997.
- Price, Nicholas and Dwek, Raymond and Wormald, Mark. Principles and Problems in Physical Chemistry for Biochemists. R. G. Ratcliffe. New York: Oxford University Press, 1997.

