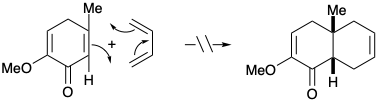4.9: Study Questions
- Page ID
- 285645
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
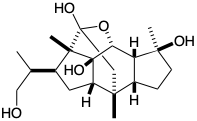
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)1. Circle the "common atoms" in cincassiol D1, a diterpene, and then draw wavy lines through each C-C bond that involves at least one common atom and that lies on a consonant circuit (refer to your answer to question 4 in chapter 1 for a polar reactivity analysis of cincassiol D1). You may wish to use the structures below as templates for some of your drawings. Simply "white out" unwanted bonds.
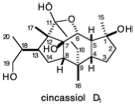
(a) For each of the dislocations suggested by your topological analysis and refering to your previous polar analysis, draw a synthon that could theoretically provide the target skeleton by polar C-C bond-forming reactions.
(b) For some of these synthons draw a functionalized precursor (synthetic equivalent) that could be used in a direct polar syntheses of the target or that could be used in a polar synthesis requiring addition of a methyl group and/or formation of a a heterocyclic C-O bond after completion of the multicyclic carbon skeleton by polar C-C bond formation.
(c) For the remaining synthons which contain consonant circuits that are blocked by quaternary centers, synthetic equivalents exploiting the polar activation afforded by target-related functional groups are not available since conjugation of these functional groups with one or both of the reacting carbon centers is not readily achieved without prior cleavage of additional carbon-carbon bonds. Label these synthons as "BLOCKED".
2. The deduction of likely biosyntheses of terpenes provides an excellent opportunity to practice retrosynthetic analysis with a set of boundary conditions: (a) isopentenyl pyrophosphate as starting material, (b) head to tail acyclic oligomer as an intermediate, (c) carbocationic electrophile and C=C π-bond nucleophile for C-C bond formation, (d) all of the reactions of carbocation intermediates as potential steps, e. g. β-proton loss, and rearrangements by 1,2-C shifts or hydride shifts.
(a) Deduce likely biosyntheses for the following regular sesquiterpenes:
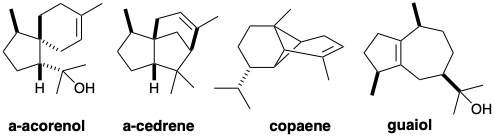
Hint: notice and think about any similarities in the structures of α-acorenol and α-cedrene.
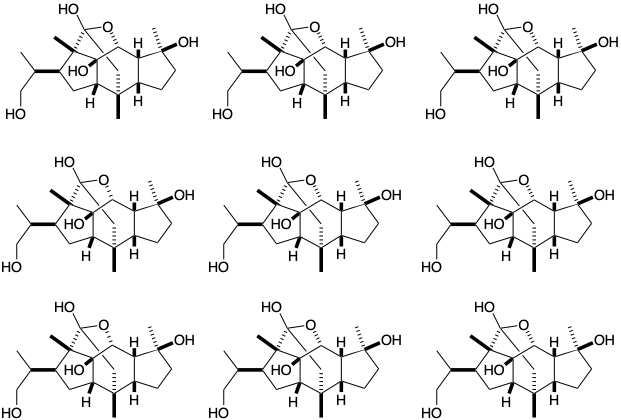
(b) Cincassiol D1 is a regular diterpene. In the following structure: (a) circle the isoprene units, (b) draw wavey lines through all C-C bonds that are not bonds within the isoprene units, (c) for those nonisoprene bonds that would be present in the acyclic geranylgeranyl pyrophosphate precursor of cincassiol D1, label the ends with h or t to indicate a head or tail atom of an isoprene unit.
(c) Draw a circle around each of the “common atoms” (as defined by Corey) in the following structure of lanosterol (1).
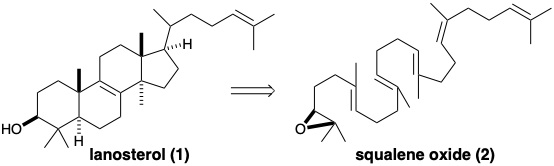
(d) Describe the topological strategy of the biosynthesis of lanosterol (1) from squalene oxide (2).
(e) Write a mechanism for the polyene cyclization and rearrangement involved in the biosynthesis of lanosterol (1) from squalene oxide (2). Show the flow of electrons with arrows pointing from electron pairs in the reactants to their impending location in the incipient products. Show the polyene cyclization as a single concerted process involving several electron pairs and leading to an intermediate cation. Then show the rearrangement of that intermediate to lanosterol as another concerted process involving the movement of several electron pairs.
3. By drawing the appropriate resonance forms of a key cationic and a key anionic intermediate, write a mechanism that shows how the C-C bond forming reaction between 3 and 4 generates 5 by a process that relies upon the stabilizing influence of functionality in 3 and 4 that is related to the 3-hydroxyl and 17-keto groups in the final synthetic target, estrone (6).
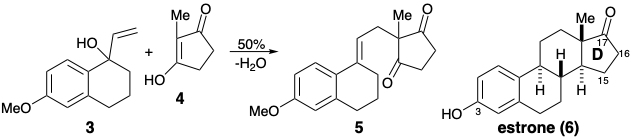
Hint: 4 is a vinylogous carboxylic acid.
4. For each of the following reactions that generate a trisubstituted alkene stereoselectively, what is the driving force and why is it stereospecific?
(a) 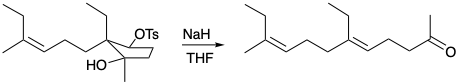
(b) 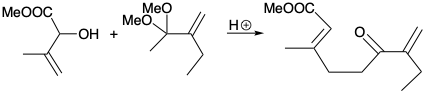
(c) 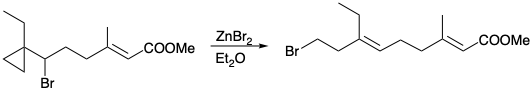
5. A remarkable reaction occurs upon UV irradiation of 7 and 8. A product 9, that contains the loganin skeleton is generated stereoselectively.
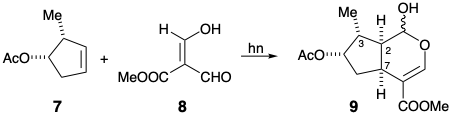
(a) Write a mechanism for this reaction. Show the flow of electrons with arrows pointing from electron pairs in the reactants to their impending location in the incipient products.
(b) Use one or more of the following terms to answer each of the following questions: thermodynamic control, stereoelectronic control, steric approach control, or temporary bridge. How is stereocontrol achieved: (i) at the 3 position relative to the 2 position in 9? (ii) at the 7-position relative to the 2-position in 9?
6. In the Kojima-Saki synthesis of PGF1α, the relative stereochemistry at positions 8, 9, and 12 is set in the conversion of 10 into 11.
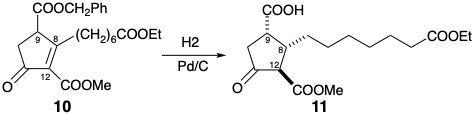
How is stereocontrol achieved: (i) at the 12 position relative to the 9 position in 11? (b) at the 8- position relative to the 9-position in 11?
7. Each of the following reactions fails because of a fatal flaw. For each reaction draw the structure of the actual product.
(a) 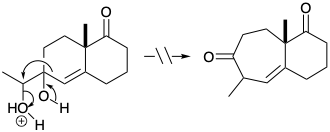
(b) 
(c) 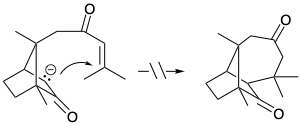
(d)