4.3: Biosynthesis of Sesquiterpenes - Longifolene
- Page ID
- 285451
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The sesquiterpenes are C15 compounds derived biogenetically from E,E-farnesyl-PP (8), the allylic isomer nerolidyl-PP (22), or the geometric isomer Z,E-farnesyl-PP (23). Nucleophilic attack by a C=C π-bond on the electrophilic pyrophosphate generates various isomeric cationic intermediates such as 24-31 which undergo proton loss, nucleophilic capture by external nucleophiles (especially water) or by another C=C π-bond to generate a wide variety of carbon networks.

Retrosynthetic analysis of the biosynthesis of the sesquiterpene longifolene (32) is channeled by the boundary condition that the starting material most probably is a head-to-tail-head-to-tail trimer of isopentenyl pyrophosphates. The longifolene skeleton is an intricate network of carbon. The analysis must simplify the tricyclic topology by disconnections which generate or lead to an acyclic precursor such as Z,E-farnesyl-PP (23). Three isoprene units are clearly discernable embeded in the skeleton of longifolene. Unmasking of the acyclic trimeric starting material requires disconnection of some bonds between these isoprene units. A series of disconnections of C=C π-bond nucleophiles from carbocationic electrophiles can be achieved by proton addition to 32 to give 33. Retropolyene cyclization of 33 disconnecting a nonisoprenoid bond suggests the precursor 34. Similar disconnection of this carbocationic intermediate suggests a precursor 35, but further disconnection of nonisoprenoid bonds cannot proceed from this carbocationic precursor. Therefore, hydride migration producing an isomeric carbocation must follow the cyclization that generates the carbon skeleton of 35. The isomeric carbocation 36, on the other hand, can be generated by addition of a carbon electrophile to a C=C bond in 37 which has the carbon skeleton of a head-to-tail-head-to-tail isoprenoid trimer. Intermediate 37 could be generated from Z,E- farnesyl-PP (23) by elimination of pyrophosphoric acid and subsequent addition of a proton to an intermediate tetraene 38.
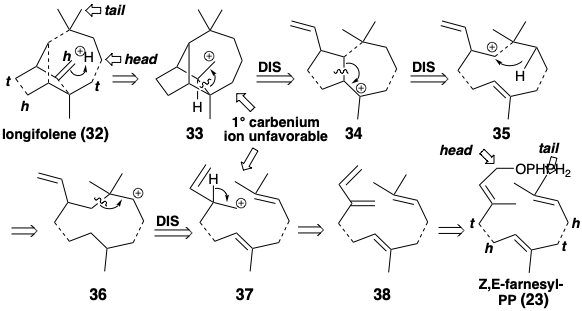
The actual biosynthetic strategy for longifolene (1) is similar to that inferred above but avoids generating relatively unstable 1° carbenium ions such as 33 or 37 by exploiting a skeletal rearrangement step. Such carbenium ion rearrangements are a common occurrence during the biological construction of carbon networks, particularly those of many terpenes. Addition of the allylic electrophile to a nucleophilic trisubstituted C=C π-bond in 23 generates 29 or 31 that can rearrange to a more stable 2° allylic carbenium ion 39 by 1,3-hydride shift. Cationic polyene cyclization then delivers a bicyclic 3° carbocation 40 and then tricyclic 2° carbocation 41 that undergoes [1.2] sigmatropic rearrangement of carbon, a Wagner-Meerwein rearrangement, to produce a more stable 3° carbocation 43 with the longifolane skeleton. The 41 to 42 rearrangement is readily reversible (vide infra). Deprotonation of 42 delivers longifolene.

The biosyntheses of all the multicyclic sesquiterpenes involve similar carbocationic polyene cyclizations. Channeling the cyclization to specific structures is undoubtedly influenced by the folding of the acyclic pyrophosphate substrate by various protein catalysts (enzymes) promote the reactions and also limit the access of water to the carbocationic intermediates. Otherwise, the carbocationic intermediates would be captured by water to produce various alcohols resulting from interception of the numerous intermediates. It is also possible that folding causes juxtapositions of p-bonds that favor a concerted formation of several sigma bonds without the generation of numerous discrete carbocationic intermediates such as those shown in the above scheme for the biosynthesis of longifolene.



