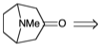2.5: Study Questions
- Page ID
- 285439
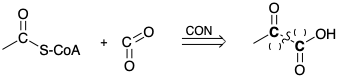
1. Different strategies are adopted in Nature for the disconnection of \(\ce{CO2}\) from acetylCoA during the biosynthesis of acetylCoA and for the connection of \(\ce{CO2}\) to acetylCoA during the biosynthesis of malonylCoA from acetylCoA.
(a) Indicate with (+) or (-) next to each C in the following retrosynthetic analysis to show the polar activation provided by the carboxyl functionality for the carboxylation of acetylCoA.
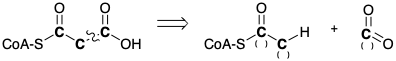
(b) Indicate the functionality levels of each atom in boldface type in the following strategy for the direct synthesis of malonylCoA from acetylCoA.
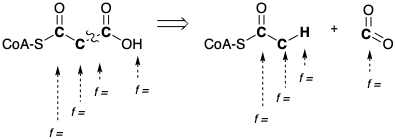
(c) Indicate with (+) or (-) next to each C in the synthon to the left the appropriate polar reactivity required for a strategy which generates acetylCoA by direct cleavage of \(\ce{CO2}\) (decarboxylation) of a precursor.
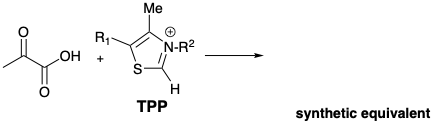
(d) Draw an intermediate, derived from pyruvic acid and thiamine pyrophosphate (TPP), that is a synthetic equivalent of the above synthon. With (+) and (-) next to the appropriate atoms, indicate the polar activation provided by functionality in the intermediate that allows the polar decarboxylation. Show the bond that cleaves in the biosynthesis with a wiggly line.
2. Tropinone is a key degradation product obtained during determination of the structure of atropine, a natural product of the "alkaloid" family.
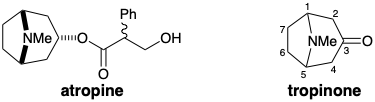
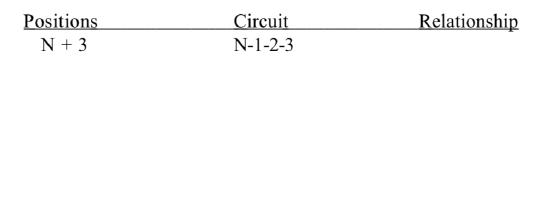
(a) Perform a thorough polar analysis of tropinone. List the polar relationships between functional groups by completing the following table:
(b) Write a retrosynthetic analysis that provides a synthetic strategy for tropinone from acyclic symmetrical starting materials each containing no more than five contiguous carbons and using only polar bond-forming reactions. In your analysis show pertinent polar reactivity patterns with (+) and (-) and indicate disconnections with wavey lines through the bond to be severed in the dislocation of the target to its precursor. If you draw a synthon, label it as a "synthon" and also draw an appropriate synthetic equivalent and label it as a "synthetic equivalent". All of the starting materials must be symmetrical!