2.3: Acetyl CoA - a Sugar-Derived Starting Material
- Page ID
- 285437
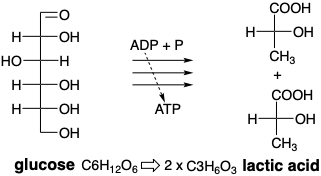
All of the carbon atoms of glucose are bound to oxygen. In contrast, many complex natural products are far less oxygenated. For example, fatty acids (Chapter 3) are long straight chains of often more than a dozen carbon atoms with no oxygen at all except for one terminal carbon that is fully oxidized to a carboxylic acid. Glucose catabolism (breakdown) can proceed anaerobically (doesn't require oxygen) producing biosynthetically useful small molecules in which some carbon atoms are less and some more highly oxidized. No net oxidation occurs. The oxygen atoms of glucose are merely reshuffled. The end product of the process is lactic acid, a molecule that is oxygen rich at one end and oxygen poor at the other. Perhaps most importantly for living organisms, anerobic catabolism of glucose also generates chemical energy in the form of ATP that can be used, inter alia, to power muscular contractions.
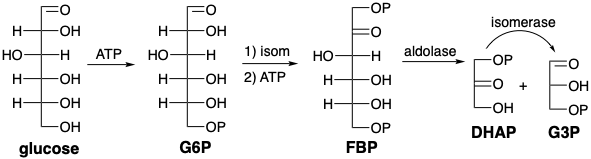
Glycolysis begins with phosphorylation of glucose followed by isomerization to fructose 6- phosphate (F6P) that is then phosphorylated further. Fructose bisphosphate is then cleaved under the influence of aldolase in a retero-aldol reaction to yield DHAP and G3P. Isomerization of DHAP then produces a second molecule of G3P.
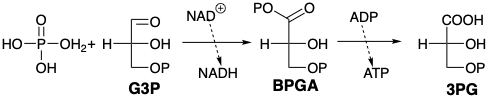
The final transformation of G3P into lactic acid begins with the removal of hydride from the aldehyde portion of the molecules (oxidation) by nicotinamide-adenine dinucleotide (NAD\({}^\oplus\)). The reaction is catalyzed by the enzyme glyceraldehyde 3-phosphate dehydrogenase. The enzyme binds G3P as a hemithioacetal that readily transfers hydride to an enzyme-bound NAD\({}^\oplus\). The product, a reactive thioester of phosphoglyceric acid, readily acylates phosphate to yield bisphosphoglyceric acid (BPGA), a reactive mixed anhydride. BPGA then phosphorylates ADP. Hence, the chemical energy generated in this oxidation is stored in the phosphate bond energy of ATP.
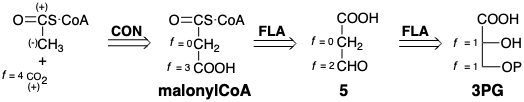
For the biosynthesis of fatty acids (Chapter 3), terpenes (Chapter 4), or polyketides (chapter 5), phosphoglyceric acid is dismantled further to form a molecule of \(\ce{CO2}\) and a thioester of acetic acid with a structurally complex thiol, coenzyme A. This thioester, referred to as acetyl CoA, has one carbon that is completely reduced, a methyl group. Since the acetyl methyl is potentially nucleophilic and the carbon of \(\ce{CO2}\) is electrophilic, an obvious strategy for the biosynthesis of acetyl CoA and \(\ce{CO2}\) from 3PG uses malonyl CoA as the penultimate target and exploits polar cleavage of a C-C bond during decarboxylation. Adjustment of the functionality level of this subtarget suggests a precursor 5 which has the same overall functionality level as the desired starting material 3PG. Conversion of 3PG to 5 could be achieved by elimination of water followed by readdition and hydrolysis of the phosphate. Interestingly, this strategy is not used for the biosynthesis of acetyl CoA although the first dislocation is used, albeit in reverse, for generating malonyl CoA from acetyl CoA (vide infra).
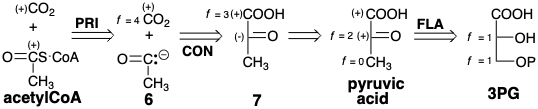
An alternative strategy for biosynthesis of acetyl CoA from 3PG involves cleavage of \(\ce{CO2}\) from the incipient acyl carbon. But this requires umpolung of the normal electrophilicity of the carbonyl carbon in acetyl CoA. That is, polar cleavage would have to generate an umpoled acyl nucleophile synthon 6 from an umpoled synthon 7 of pyruvic acid. Generation of pyruvic acid from 3PG only requires redistribution of functionality. This is the actual biosynthetic strategy for acetyl CoA.
The biosynthesis of acetyl CoA uses many of the reactions involved in the anerobic catabolism of glucose. Therefore, let us resume our discussion of the biosynthesis of lactic acid from glucose (see above). The 3-phosphoglyceric acid (3PG) that results from oxidation of G3P (see above) undergoes a transfer of the phosphoryl group from the 3- to the 2-hydroxyl and subsequent dehydration to phosphoenolpyruvate (PEP). This enol ester is energy rich since its hydrolysis generates a relatively strong C=O bond at the expense of a relatively weak C=C bond. PEP readily phosphorylates ADP releasing pyruvic acid. The carbon atom of the ketone carbonyl group of this α-ketoacid is very electrophilic and readily accepts hydride from NADH under catalysis of the enzyme lactate dehydrogenase.
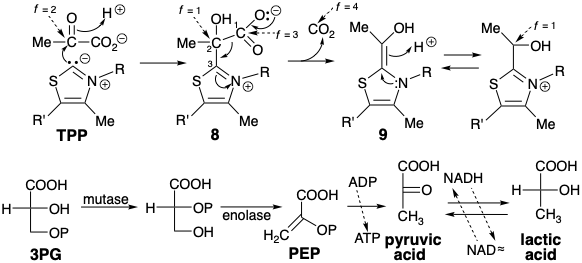
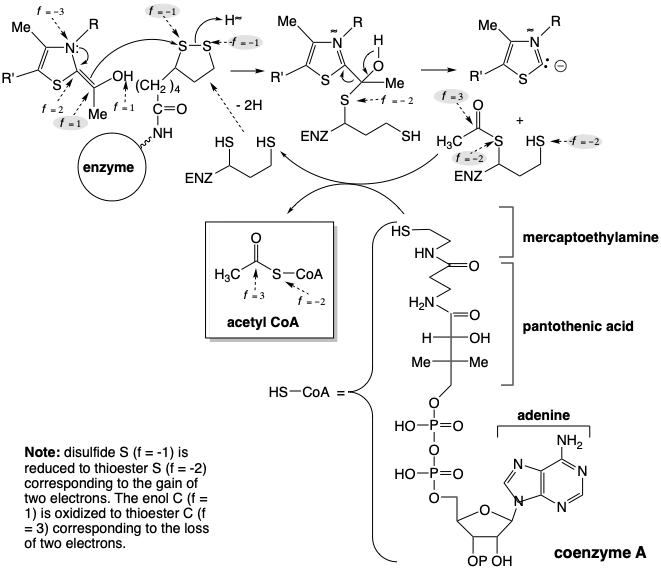
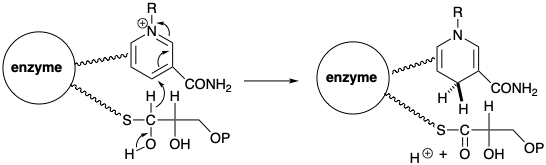
The biosynthesis of acetyl CoA from glucose involves decarboxylation of pyruvic acid that is regenerated by dehydrogenation of lactic acid. To allow a polar decarboxylation reaction, pyruvic acid mustistransformedbyumpolungoftheketonecarbonylintoamorereadilydecarboxylatedderivative. As in the transketolase reaction, the polar reactivity of this carbonyl group is temporarily inverted by the biphilic thiazole carbanion moiety of thiamine pyrophosphate (TPP). Thus, the nucleophilic thiazole ring carbon of TPP condenses with the highly electrophilic carbonyl carbon of pyruvic acid to give an adduct 8 that resembles a β-ketoacid. This undergoes decarboxylation by retro Claisen cleavage to deliver hydroxyethylidene TPP (9). The functionality level of the incipient carboxyl carbon is only f = 1 in 9. Therefore, oxidation of 9 is required to produce an acetyl functionality level (i. e. f = 3).
This oxidation is achieved by a polar process that concomitantly reduces a disulfide to a dithiol. Thus, 9 transfers an acetyl group to the disulfide of a lipoic acid residue bound to the enzyme dihydrolipoyl transacylase. The acetyl group is then transferred from the enzyme bound thiol to the thiol group of a coenzyme (CoA) to give acetyl CoA.