2.1: Carbon Fixation - Biosynthesis of Sugars
- Page ID
- 285435
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Strategies for Glucose Biosynthesis
Glucose is the ultimate organic starting material from which all other organic carbon compounds can be synthesized in nature. The single carbons of six carbon dioxide molecules are stitched together to form glucose by photosynthetic organisms. The energy for this reaction is provided by hydrolysis of adenosine triphosphate (ATP) to produce adenosine diphosphate (ADP) and phosphate (P). Hydrogen atoms are provided by the 1,4-dihydro derivative (NADPH) of nicotinamide-adenine dinucleotide phosphate (NADP+) and by protons. NADPH is a source of hydride, a "hydride transfer agent", that is stable in the aqueous environment of biosynthesis, i.e. at physiological pH it does not react with protons to generate molecular hydrogen. The high energy triphosphate ATP is produced by a photochemical phosphorylation of ADP to yield ATP. The reducing agent NADPH, a dihydropyridine, is produced in the same reaction by photochemical reduction of NADP+. Another important product of this reaction is molecular oxygen that is needed for the oxidative catabolism of natural products to provide energy in the form of ATP, i.e. by oxidative phosphorylation of ADP.


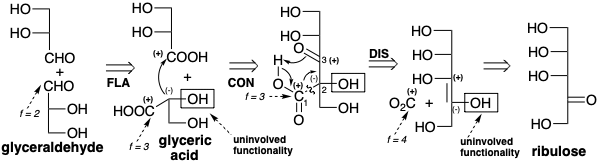
Elongation of a five carbon sugar chain to a six carbon chain by appending a molecule of the one carbon electrophile \(\ce{CO2}\) is an especially obvious strategy for a synthesis of glucose from \(\ce{CO2}\). Addition of a carbon nucleophile to \(\ce{CO2}\) (f = 4) would produce a carboxyl group (f = 3). This suggests that the first dislocation of the target might be oxidation of the aldehyde group (f = 2) to a carboxyl. There is a consonant circuit between the carboxyl group and the oxygen functionality in position 3 of the resulting gluconic acid subtarget. However, expression of the requisite polar reactivity at the 2-position requires conjugation as in the enol of ribulose. Therefore, adjustment of the functionality level at position 3 is a second logical second dislocation. Disconnection of the terminal carboxyl (a retro Claisen condensation) as the third dislocation of the target then suggests ribulose as a starting material.


Interestingly, the above strategy is not operative in the biosynthesis of glucose although it is used, albeit in reverse, for the generation of ribulose from glucose by the phosphogluconate pathway (vide infra). Rather, the biosynthesis of glucose involves a different strategy although the starting materials are indeed \(\ce{CO2}\) and ribulose. Glucose has ample functionality to facilitate its construction by C-C connective strategies involving generation of any of its C-C bonds by polar reactions. For example, the 3,4-bond could be generated by reaction of a nucleophilic synthon corresponding to carbons 1-3 with an electrophilic fragment corresponding to carbons 4-6. This suggests a first dislocation of the target involving adjustment of functionality level to allow conjugation of the C-2 oxygen with position 3 to facilitate generation of nucleophilic reactivity at C-3. By coupling oxidation at the 2-position with reduction at the 1-position, the first dislocation is simply an isomerization of the target glucose, an aldose, to fructose, a ketose. Polar disconnection of the subtarget (a retro aldol condensation) in the second dislocation has the important consequence of dividing the target into two similarly functionalized fragments with identical carbon skeletons. Such dislocations potentially reveal latent symmetry which is defined as the possibility of deriving two halves of a target from a common starting material. The precursors generated in the second dislocation, dihydroxyacetone and glyceraldehyde are readily interconverted by isomerization through an enediol intermediate.

Once again, we note that incorporation of \(\ce{CO2}\) into a precursor by a polar process will generate a carboxyl group. This suggests a carboxylic acid, glyceric acid, that can serve as a common precursor for both dihydroxyacetone and glyceraldehyde. Reterosynthetically this involves dislocation of dihydroxy acetone to glyceraldehyde (an isomerization) and dislocation of both molecules of glyceraldehyde to the same acid precursor (oxidation). Polar connection of two molecules of glyceric acid (a Claisen condensation) suggests a β-ketoacid subtarget from which a carboxyl group can be disconnected in the last dislocation of the target leading to the precursors \(\ce{CO2}\) and ribulose.
Biosynthesis of Glucose
In fact, the actual biosynthesis involves carboxylation of this five carbon sugar, albeit in the form of a bisphosphate derivative, ribulose-1,5-bisphosphate (RuBP). A strategy for biosynthesis of the subtarget RuBP might proceed in a stepwise fashion adding one \(\ce{CO2}\)-derived carbon at a time to a growing carbon chain. Such a process might require a different enzyme to catalyze fixation of each molecule of \(\ce{CO2}\) by addition to different subtargets. However, a much more ingenious strategy is adopted in nature. Carbon fixation occurs only by the reaction of \(\ce{CO2}\) with RuBP. Therefore, only a single enzyme is required to catalyze the process. Six molecules of \(\ce{CO2}\) are combined with six molecules of the five-carbon sugar derivative RuBP to produce twelve molecules of glyceric acid two of which are used to generate glucose by the above strategy. The thirty carbons of the remaining ten glyceric acid molecules are reshuffled to regenerate six five-carbon RuBPs. Thus, RuBP also functions as a catalyst for the bioconversion of \(\ce{CO2}\) into glucose.
The photosynthetic formation of glucose (actually in "the dark reactions of photosynthesis") involves an intricate series of reactions known as the Calvin cycle. In the accompanying reaction schemes, P is used to represent a phosphate ester [P = –\(\ce{PO3^2-}\)]. The carbon fixation cycle begins with carboxylation of RuBP (see below), a reaction that is catalyzed by ribulose bisphosphate carboxylase oxidase (RuBisCO) that is probably the most abundant protein on Earth. Thus, carboxylation of the enol of RuBP leads to a presumed b-keto acid intermediate that is readily cleaved by water in a retro Claisen condensation to give two molecules of 3-phosphoglyceric acid (3PG). Given the importance of this chemistry for the biosynthesis of organic molecules and the success of carbon-based life forms, it is noteworthy that RuBisCo catalysis of this reaction is barely viable. At ambient levels of carbon dioxide and oxygen, the catalyst consumes only a few molecules of \(\ce{CO2}\) per second in contrast with many enzymes that process thousands or tens of thousands of molecules of substrate per second. Furthermore, RuBisCO catalyzes another reaction that competes with carboxylation, the oxidative cleavage of RuBP to 3-PG plus 2-phosphoglycolate. This oxidative cleavage presumably involves electron transfer from than enolate intermediate to oxygen to produce superoxide and a cation radical. Bond formation between these two radicals then generates a hydroperoxy alkoxide. Fragmentation of this intermediate is driven by the exothermic generation of two carbonyl groups. The ratio of carboxylation versus oxidative cleavage is only about 4 to 1 and is even less favorable at higher temperatures. Thus, plants in high heat environments store carbon dioxide during the hot hours of intense sunshine, and generate glucose in the cooler hours in the absence of sunshine and its blistering heat.

The biosynthesis of all sugars, including glucose and the regeneration of RuBP, use 3PG as the common and only starting materisl. 3PG is first reduced to an aldehyde, glyceraldehyde-3-phosphate (G3P), by NADPH. ATP facilitates the reduction by converting the carboxyl into a more electrophilic derivative, a carboxylic-phosphoric anhydride. The remainder of the reactions of the Calvin cycle redistribute the thirty six carbon atoms of twelve G3Ps to yield one molecule of glucose (six carbon atoms) and regenerate six molecules of RuBP (thirty carbon atoms). The cycle will be summarized in Chart 1 below.

The aldose G3P is transformed to the ketose dihydroxyacetone phosphate (DHAP) under the influence of the enzyme isomerase. The six carbon atom skeleton of glucose is then assembled by an aldol condensation of G3P with DHAP. The initial product, fructose bisphosphate (FBP), is hydrolyzed (to F6P), isomerized (to G6P) which is hydrolyzed further to yield glucose.

Regeneration of Ribulose Bisphosphate
Retrosynthetic analysis reveals that a polar synthesis of Ru5P from G3P requires an umpoled synthon, the 2-hydroxyacetyl carbanion.  A boundary condition, the aqueous reaction conditions of biosynthesis limit the choice of synthetic equivalents for this synthon. It is instructive to consider that a similar synthon 1 is required in the benzoin condensation, a cyanide ion- catalyzed polar coupling of two electrophilic benzaldehyde carbonyl groups that can be achieved in aqueous solution. Cyanide inverts the polar reactivity of a benzaldehyde carbonyl carbon by nucleophilic addition followed by a proton transfer that generates the carbanion 2, a synthetic equivalent of synthon 1. Anion generation at the former carbonyl carbon is favored by conjugation with the nitrile in 2. It is the biphilicity of cyanide that is the basis of its ability to invert the polar reactivity of an aldehyde carbonyl carbon. Condensation of 2 with a second molecule of benzaldehyde delivers alkoxide 3 which affords alkoxide 4 by proton transfer. Expulsion of cyanide from 4 then regenerates the catalyst and produces benzoin. Analogous cyanide-catalyzed reactions of other aldehydes are generically called benzoin condensations. All of the steps of the benzoin condensation are reversible. Therefore, the umpoled synthon 2 not only can be generated by reaction of cyanide with benzaldehyde, but also by retro benzoin condensation of benzoin.
A boundary condition, the aqueous reaction conditions of biosynthesis limit the choice of synthetic equivalents for this synthon. It is instructive to consider that a similar synthon 1 is required in the benzoin condensation, a cyanide ion- catalyzed polar coupling of two electrophilic benzaldehyde carbonyl groups that can be achieved in aqueous solution. Cyanide inverts the polar reactivity of a benzaldehyde carbonyl carbon by nucleophilic addition followed by a proton transfer that generates the carbanion 2, a synthetic equivalent of synthon 1. Anion generation at the former carbonyl carbon is favored by conjugation with the nitrile in 2. It is the biphilicity of cyanide that is the basis of its ability to invert the polar reactivity of an aldehyde carbonyl carbon. Condensation of 2 with a second molecule of benzaldehyde delivers alkoxide 3 which affords alkoxide 4 by proton transfer. Expulsion of cyanide from 4 then regenerates the catalyst and produces benzoin. Analogous cyanide-catalyzed reactions of other aldehydes are generically called benzoin condensations. All of the steps of the benzoin condensation are reversible. Therefore, the umpoled synthon 2 not only can be generated by reaction of cyanide with benzaldehyde, but also by retro benzoin condensation of benzoin.


A similar retro benzoin condensation of fructose 6-phosphate (F6P), an intermediate generated in the biosynthesis of glucose from glyceraldehyde 3-phosphate (G3P), could provide a synthetic equivalent of the 2-hydroxyacetyl carbanion required for biosynthetic regeneration of RuBP from G3P. In fact, the biosynthesis of RuBP from G3P involves the transfer of a 2-hydroxyacetyl group from F6P to G3P that is promoted by the enzyme transketolase.

Needless to say, cyanide is not the cocatalyst which masks the usual electrophilic reactivity of a carbonyl group and imparts nucleophilic reactivity to it in nature. The biological equivalent of cyanide ion is a carbanion generated by deprotonation of the thiazole ring in thiamine pyrophosphate (TPP).

The thiamine carbanion nucleophile condenses with the electrophilic carbonyl carbon of a ketose (e.g. F6P) to yield a 2-hydroxy iminium derivative. The latter readily undergoes a retro aldol-like reaction leading to an aldose, e.g. erythrose 4-phosphate (E4P), that has two carbons less than the original ketose. The resulting nucleophilic 2-hydroxyacetyl equivalent, 2-(1,2-dihydroxyethylidene)thiamine pyrophosphate (DETPP) can condense with a different aldose, e.g. G3P, to regenerate TPP and a ketose, e.g. xyulose 5- phopsphate (Xu5P) that has two carbons more than the aldose.
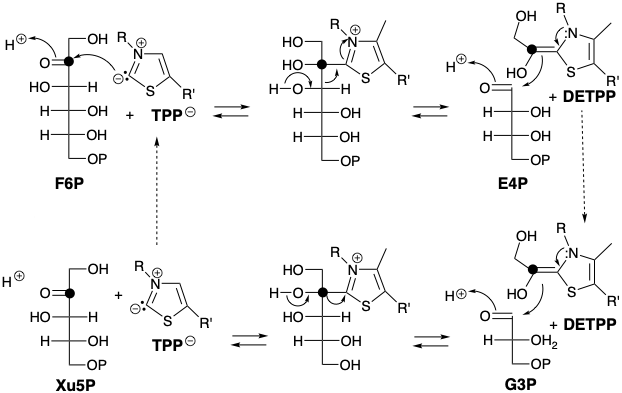

Further reactions in the Calvin cycle are aldolase promoted condensation of E4P with DHAP to yield sedoheptulose bisphosphate (SBP), hydrolysis of the latter to the monophosphate (S7P), transketolase promoted hydroxyacyl transfer from S7P to G3P to give Xu5P plus ribose 5-phosphate (R5P), isomerization of the latter to ribulose 5-phosphate (Ru5P), epimerization of Xu5P to give Ru5P, and phosphorylation of the latter to regenerate RuBP. The Calvin Cycle is summarized in chart 1.

Summary of Biosynthetic Carbon Fixation
(1) There is only one reaction that converts carbon dioxide into organic starting materials: the generation of two 3PGs from RuBP and \(\ce{CO2}\). This is step #1 in the biosynthesis of all natural products. (2) RuBP serves as a catalyst in a cycle that converts six \(\ce{CO2}\) into one molecule of glucose. (3) The RuBP consumed in step #1 is regenerated by a series of reactions, that reshuffle the atoms of ten molecules of the three-carbon sugar G3P into six molecules of the five- carbon RuBP. (4) All C-C bond formation and cleavage involves condensations (aldol, Claisen, benzoin) that are readily reversible. Furthermore, aldolase, the enzyme that catalyzes the formation of glucose from three-carbon sugars, also catalyzes their regeneration. As we shall see, further biosynthetic transformations of glucose into fatty acids, terpenes, or polyketides begin with cleavage of glucose (glycolysis) by this retro aldol reaction.
Ribulose from Glucose
Before proceding with an examination of strategies for the synthesis of glucose starting materials, let us first return to the simple strategy outlined above that is not used for the biosynthesis of glucose. Thus, rather than serving as a synthetic route to glucose from RuBP, this strategy in reverse is used in nature to produce five-carbon sugars from glucose. This pathway for glucose degradation is important for biosynthesis because it generates pentoses for the synthesis of nucleic acids. The pathway begins with oxidation of glucose 6-phosphate to 6-phosphogluconate. It is known as the phosphogluconate pathway, the hexose monophosphate shunt, or the pentose phosphate pathway. Many of the enzymes and reactions of this pathway are also involved in the biosynthesis of glucose from \(\ce{CO2}\) in the dark reactions of photosynthesis. The phosphogluconate pathway produces 2NADPH + CO2 + R5P from glucose and 2NADP+. Four enzymes are required: [1] 6-glucose phosphate dehydrogenase, [2] lactonase, [3] 6-phosphogluconate dehydrogenase, and [4] phosphopentose isomerase.



