1.2: Functional Groups, Hybridization, Naming
- Page ID
- 81768
Representation Answers
First, let's look at the various structural representations you were asked to develop for the molecules given at the end of the last lecture. Here they are:

Compare your answers with these representations. Please see me if there are puzzles.
Naming
Naming organic compounds is a necessity, and the names of large molecules can be fairly complex. The rules are simple - but picky. You can learn the basics of these rules by studying on your own using the appropriate sections in Brown. The principles are outlined in Section 3.5. The application of these rules to aldehydes and ketones are discussed in Section 11.2. Study these, practice applying them by doing problems, and bring up puzzles in class for discussion. There will be naming questions on the exams, so you will profit by working on naming. These questions will not tackle complex examples.
Shape
Now let's look at the carbonyl group so as to understand why it is a site for chemical reactivity. We'll start by examining its geometry, specifically the bond angles around the carbon atom. We'll use formaldehyde as our example:
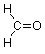
In the context of VSEPR theory we notice that there are three groups of electrons associated with that carbon, two single bonds to hydrogen and one double bond to oxygen. The best way for these clusters of negative charge to be as far apart from each other so as to minimize their mutual repulsion is to adopt bond angles of approximately 120o:
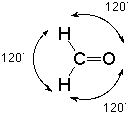
Such an arrangement is given the name "trigonal."
The problem with this is that if we think of the orbitals used by the valence electrons of a carbon atom, they are the 2s and 2p orbitals. You will remember that the 2p orbitals are arranged at 90o angles from each other.
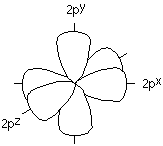
This doesn't fit the 120o bond angles we need for our carbonyl group.
Hybridization
Fortunately, the theory of quantum mechanics tells us that we can mix the 2s and 2p orbitals in suitable proportions without violating the mathematical rules of differential equations. If we mix the 2s orbital and two of the 2p orbitals in this way, the resulting orbitals are pointed at from each other. Orbitals formed in this way are called hybrid orbitals and the process is called hybridization. These particular hybrid orbitals are called sp2 orbitals since they are made by hybridizing one "s" orbital and two "p" orbitals and they have the appropriate geometry for a trigonal carbon atom such as is found in the carbonyl group. (See Section 1.14 in Atkins and Carey for the same ideas applied to the doubly bonded carbons in ethylene.)
To summarize these connections, when we see a carbon involved in a double bond, its geometry will be trigonal, with 120o bond angles, and it will have sp2 hybridization. In a shorthand way trigonal and sp2 are synonyms.
Bonding
The sp2 hybrid orbitals formed in this fashion form single bonds. In the case of formaldehyde, each bond to hydrogen is formed by overlap between the sp2 hybrid orbitals on the carbonyl carbon and 1s orbitals on the hydrogen. This forms what is called a molecular orbital, one which involves atomic orbitals from two or (less commonly) more atoms. When two electrons, usually one from each atom, occupy this molecular orbital, we have a covalent bond. This bond is referred to as a sigma bond since it is shaped like a cylinder (hot dog) and the letter sigma is the first letter of the greek word for cylinder.
In a similar way, one of the bonds beween the carbon and the oxygen is formed by overlap of an sp2 hybrid orbital from carbon and a similar orbital from oxygen. (The oxygen orbital is also an sp2 hybrid orbital, but we will not pursue that.) This is also a sigma bond. What about the second carbon-oxygen bond?
When we discussed the hybridization process earlier, you may have wondered what happened to the carbon 2p orbital which wasn't used to form the sp2 hybrid orbital. It is still there, and it is used to make the second bond to oxygen. It overlaps with a similar 2p orbital from the oxygen atom to form what is called a pi bond. The term pi is used because half of this bond extends above the plane of all three sigma bonds and half extends below that plane. The first letter of the greek word for plane is pi. The pi molecular orbital can be visualized as a hot dog bun, which would lead us to visualize the double bond as a complete hot dog. One bond is a sigma bond, represented by the sausage, and the other is a pi bond, represented by the bun.
This description of the bonding in formaldehyde can be represented this way (see also Figure 11.1 in Atkins and Carey):
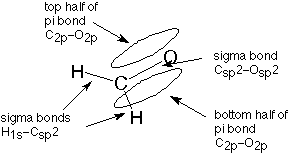
Similar representations apply to the double bonded carbons in ethylene (Atkins and Carey, Section 1.14) and to carbons involved in one double bond wherever they are found.
Reaction Sites
Why does this bonding scheme make the carbonyl group a reactive group, a functional group? There are three things involved here, at least one of which is seen in every functional group.
First, notice that the pi bond is above and below the plane of the sigma bonds. That means that the electrons in this bond (the pi electrons) are not located between the positively charged carbon and oxygen nuclei, but are instead above and below a line joining them. The pi electrons are farther away from the nuclei and are less strongly held to them than are sigma electrons. These pi electrons are consequently easier to move into a different location than are sigma electrons. If they are moved so as to make a new bond involving another atom, the pi bond has been broken, the structure has changed, and a reaction has occured. The short statement: pi bonds are easier to break than sigma bonds. We'll look at several examples in the next few lectures.
Second, both carbon-oxygen bonds are polar (see Atkins and Carey, Sections 1.5 and 11.2). That means that the electrons in both bonds are more strongly attracted to the more electronegative oxygen atom than the less electronegative carbon. This results in an oxygen which is slightly negatively charged and a carbon which is slightly positively charged. An atom which has an electron pair to donate (a Lewis base) will be attracted the electron-poor carbon and will show a tendency to make a new bond there. An atom which is deficient in electrons (a Lewis acid) will correspondingly be attracted to the electron-rich oxygen and show a tendency to bond there. We can indicate this polarity using the symbol +-->, where the arrow points towards the more negative end. We can also use the greek letter delta to describe a small amount (much less than an electron's worth) of charge:
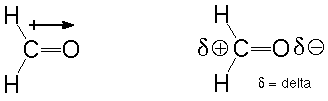
The short statement: polar bonds are places where reactions occur.
Third, there are unshared pairs of electrons (Lewis base structural sites) on the oxygen. This can be seen if we take our expanded structure for formaldehyde and convert it to a Lewis dot structure by replacing each line with two dots to represent electrons:

This picture is incomplete. The oxygen does not have a full octet and should not be neutral in a formal charge sense. We can correct this by showing the two unshared pairs on the oxygen atom. Doing so represents the full octet and the formally neutral oxygen, and most importantly, tells us that the oxygen is a Lewis base.
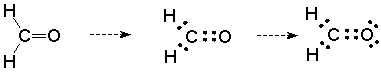
We will then expect that the oxygen will be the site of reaction with Lewis acids. This reinforces the conclusion we arrived at on the basis of polarity.
For contrast, consider that the "R-group" parts of molecules are made of carbons and hydrogens which are connected by sigma bonds. The carbons are sp3 hybridized (Atkins and Carey, Section 1.12) and cannot make pi bonds. Carbon and hydrogen have very similar electronegativity, so the sigma bond between them is not at all polar. All the electrons associated with the carbon and hydrogen atoms are involved in bonding, and each has a completely occupied valence shell. There are no unshared pairs or vacancies, so neither atom will behave as a Lewis acid or base.
To put these ideas into action, go through the functional groups listed inside the front cover of Atkins and Carey (ignore the alkanes). Each group is reactive for one or more of the reasons listed above. List the reasons for each group, and be ready to discuss any puzzling issues in class.


