2.4: TLC -ANALYSIS
- Page ID
- 135945
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Thin-layer chromatography (or TLC) is a quick, cheap, and reliable way to evaluate the composition of a sample, and the identity of a given compound. The TLC plate works by chromatographic principles: a mobile phase (solvent or solvent mixtures) will climb up the plate material (stationary phase). Compounds that are less polar will wander farther on the plate, because they experience les1s attraction to the stationary material. Compounds that are more polar will wander lower on the plate, because they experience more at- traction to the stationary material. This process is separating the different compounds present in the crude sample
Most compounds that we handle in the o-chem labs at PSU should give a single spot on the TLC plate. Ideally, each compound in a mixture will produce a distinct spot so a sample with two compounds will give two different spots, and so on.
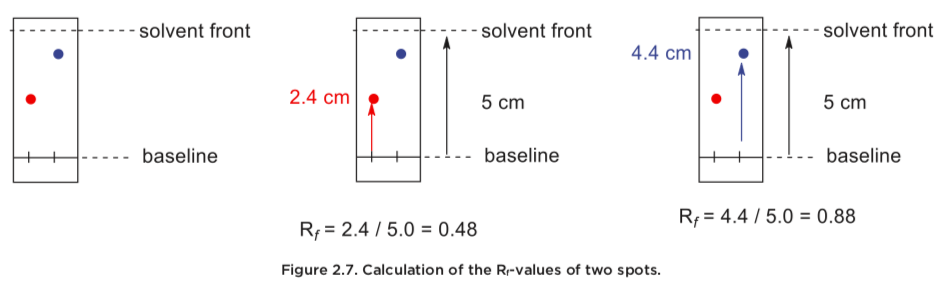
An important property of any compound, is its Rf-value (retention factor). In simple terms, this value is an indication of how far up a TLC-plate a compound has wandered. A high Rf -value indicates that the compound has travelled far up the plate and is less polar, while a lower Rf -value indicates that the compound has not travelled far, and is more polar. This value is easily calculated by measuring the distance the spot has wandered, and dividing this by the distance the solvent has traveled. The Rf-value is dependent on both the compound and the solvent used for development. If we analyze two compounds and they give the same Rf -value with the same solvent system, the two compounds are most likely identical.
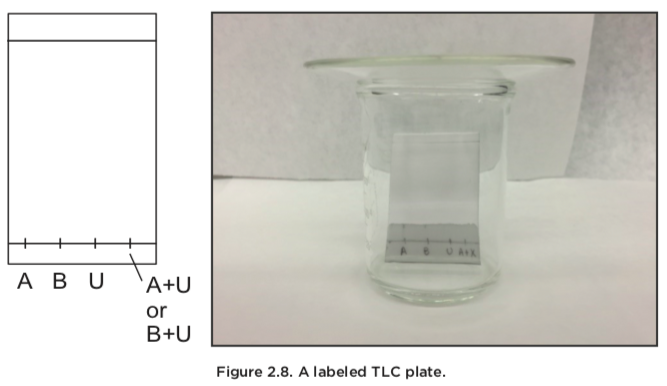
The apparatus for doing a TLC-experiment is very simple. We need a developing chamber where the mobile phase is kept. The chamber should be sealed, and although there are special TLC-chambers one can use, a beaker covered by a watch glass to create a seal often suffices.
How to prepare a TLC plate:
- A horizontal line is drawn at the bottom of the plate (5-8 mm from the bottom edge) and another horizontal line is drawn at the top of the plate (8 mm from the top edge). A gentle soft and a very soft pencil must be used because too much pressure or harder pencils will crack the very thin coating on the plate, leading to unacceptable TLC results after development (ink pens should never be used because the ink will wander up the plate).
- Vertical ticks are drawn on the bottom line, depending on how many samples are analyzed. These are usually labeled (A, B, C, for example)
- Solutions are prepared of all the samples that must be analyzed. These must neither be too concentrated, nor too dilute and a concentration of about 5% is a good starting point. The samples are applied to the ticks with capillary tubes without damaging the coating on the plate.
- The TLC plate is placed into the developing chamber, and care is taken to make sure that the bottom edge of the plate is perpendicular to the solvent. If not, parts of the plate will overdevelop, and we will get poor resolution and unclear results.
- The chamber is sealed with the watch glass and the solvent front is allowed to climb up to the topmost line. We then remove the plate and allow it to dry before we observe the plate under UV-light.
We will discuss two common ways to use a TLC- analysis. The first is to verify the identify of a com- pound. A quick TLC analysis can be used to identify whether or not an unknown compound is the same as another known compound. We typically make three applications: one of the unknown sample, one of a stock (known) compound, and a third with one spot of both (called a co-spot). If we find that the two spots have the same Rf-values, and the third spot only shows one spot, the two compounds are identical.
The second common way to use a TLC- plate, is to monitor a reaction. We can use TLC-analyses to see whether or not a reaction has gone to completion. We typically see the disappearance of the starting material(s) and the emergence of a new spot that corresponds to the product. We usually have to make controls as described above, with spots of the starting material(s) to learn their Rf-values.
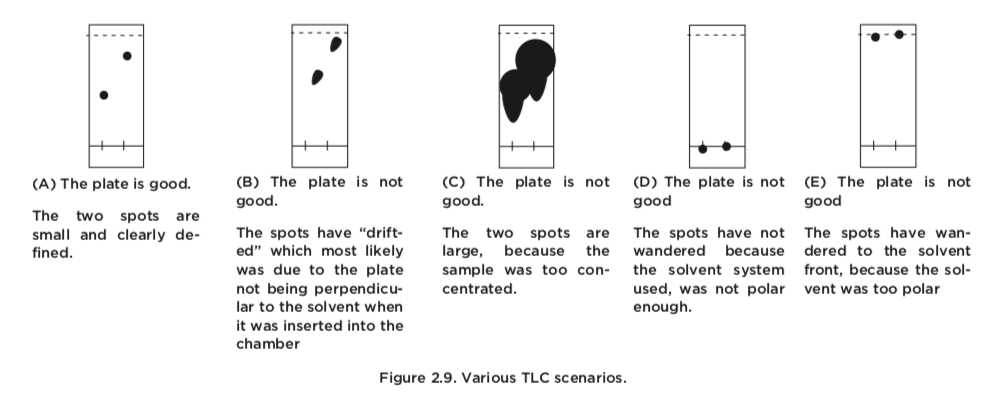
Like any of the techniques we have discussed in this chapter, TLC-analyses require a fair amount of care to get right. The most likely problems can be traced to the application and preparation of the TLC-plate. Let us have a closer look at some TLC plates that are of inadequate quality and their causes.


