2.7: Electron Configurations
- Page ID
- 86598
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe how electrons are arranged in an atom using electron configurations.
Previously we discussed the concept of electron shells, subshells, orbitals, and electron spin. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that.
We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell, which is labeled 1s and can hold a maximum of 2 electrons. We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell. Thus, because a hydrogen atom has its single electron in the s subshell of the first shell, we use 1s1 (spoken as “one-ess-one”) to describe the electron arrangement or distribution of electrons in hydrogen. This structure is called an electron configuration and is unique to hydrogen.
Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells contain one s orbital which can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s2 (spoken as “one-ess-two”).
The 1s subshell can hold a maximum of 2 electrons, so the electron configuration for a lithium atom, which has three electrons, cannot be 1s3. Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2s orbital. Therefore, we write the electron configuration of a lithium atom as 1s22s1 (spoken as “one-ess-two two-ess-one”).
The shell diagram for a lithium atom (Figure \(\PageIndex{1}\)). The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1s, while the outermost shell (2s) has 1 electron.
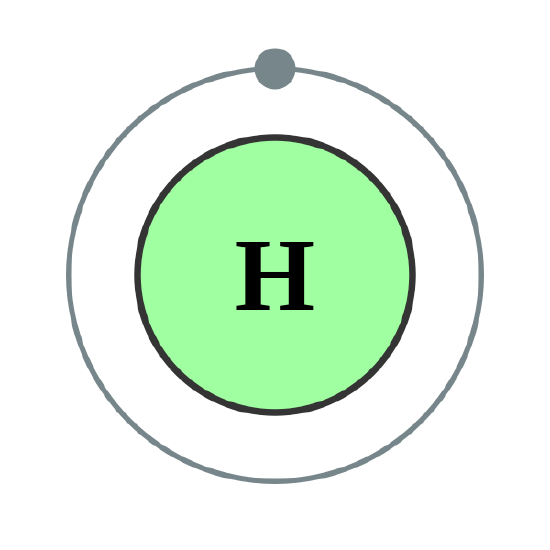
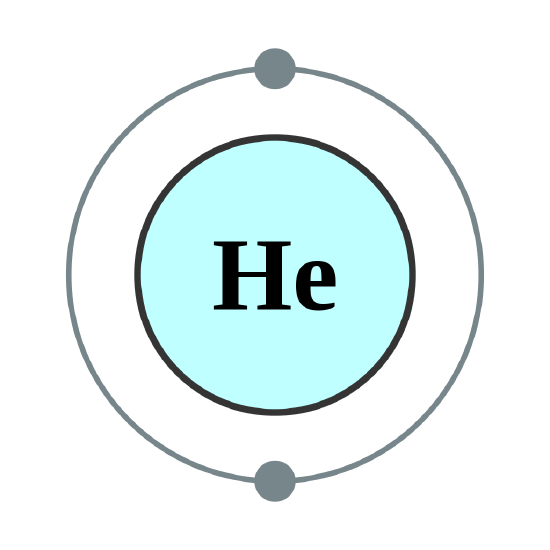
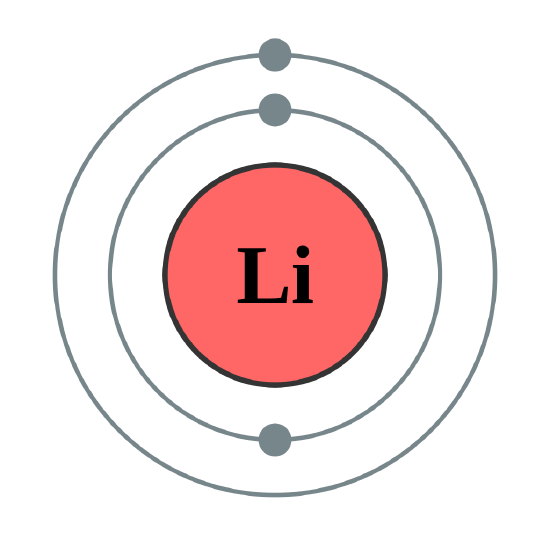
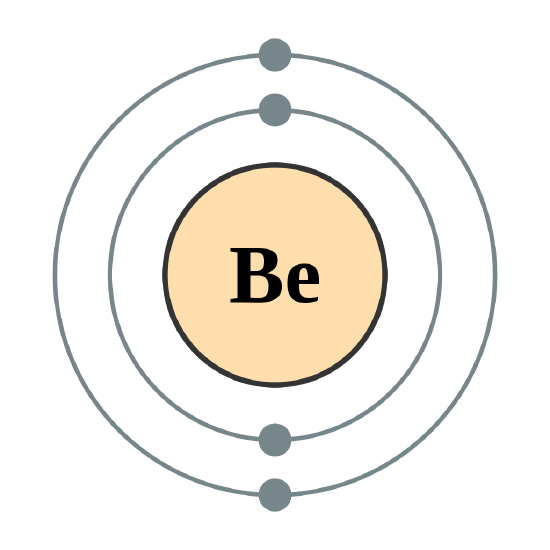
There are a set of general rules that are used to figure out the electron configuration of an atomic species: Aufbau Principle, Hund's Rule and the Pauli-Exclusion Principle.
- Rule 1 (Aufbau Principle): Electrons occupy the lowest-energy orbitals (closest to the nucleus) possible, starting with 1s, then 2s, 2p, and continuing on to higher energy (further away from the nucleus). Shells increase in energy in order from 1 to 2 to 3, and so on. Within these shells, an s subshell is the lowest energy followed by p, then d, then f.
- Rule 2 (Hund's Rule): When electrons occupy degenerate orbitals (i.e. same shell and subshell), they must first singly occupy (half-fill) each empty orbital in a subshell before double occupying (completely filling) them. Furthermore, the most stable configuration results when the spins are parallel (i.e. all spin up or all spin down). For example, all three p orbitals in a p subshell will have one electron before a single p orbital contains two electrons.
- Rule 3 (Pauli-Exclusion Principle): Each electron is described with a unique set of four quantum numbers (a unique address). Therefore, if two electrons occupy the same orbital, they must have different spins. This is the reason all orbitals can hold a maximum of two electrons.
Continuing on the periodic table to the next largest atom, beryllium, with 4 electrons, the electron configuration is 1s22s2. Now that the 2s subshell is filled, electrons in larger atoms, starting with boron, begin filling the 2p subshell, which can hold a maximum of six electrons. The next six elements progressively fill up the 2p subshell:
- B: 1s22s22p1
- C: 1s22s22p2
- N: 1s22s22p3
- O: 1s22s22p4
- F: 1s22s22p5
- Ne: 1s22s22p6
At the end of the period the element neon, has filled the 2s, and 2p subshells, which completes the second shell. Now atoms with more electrons now must begin the third shell starting with the 3s subshell. The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s22s22p63s23p1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more complicated at this point (after argon, with its 18 electrons), so we will not consider the electron configurations of larger atoms.
| Atomic Number | Element Symbol | Outermost Shell | Electron Configuration | Noble Gas Configuration |
|---|---|---|---|---|
| 1 | H | 1 | 1s 1 | 1s 1 |
| 2 | He | 1 | 1s 2 | 1s 2 |
| 3 | Li | 2 | 1s 2 2s 1 | [He] 2s 1 |
| 4 | Be | 2 | 1s 2 2s 2 | [He] 2s 2 |
| 5 | B | 2 | 1s 2 2s 2 2p1 | [He] 2s 2 2p1 |
| 6 | C | 2 | 1s 2 2s 2 2p2 | [He] 2s 2 2p2 |
| 7 | N | 2 | 1s 2 2s 2 2p3 | [He] 2s 2 2p3 |
| 8 | O | 2 | 1s 2 2s 2 2p4 | [He] 2s 2 2p4 |
| 9 | F | 2 | 1s 2 2s 2 2p5 | [He] 2s 2 2p5 |
| 10 | Ne | 2 | 1s 2 2s 2 2p6 | [He] 2s 2 2p6 |
| 11 | Na | 3 | 1s 2 2s 2 2p6 3s 1 | [Ne] 3s 1 |
| 12 | Mg | 3 | 1s 2 2s 2 2p6 3s 2 | [Ne] 3s 2 |
| 13 | Al | 3 | 1s 2 2s 2 2p6 3s 2 3p1 | [Ne] 3s 2 3p1 |
| 14 | Si | 3 | 1s 2 2s 2 2p6 3s 2 3p2 | [Ne]3s 2 3p2 |
| 15 | P | 3 | 1s 2 2s 2 2p6 3s 2 3p3 | [Ne] 3s 2 3p3 |
| 16 | S | 3 | 1s 2 2s 2 2p6 3s 2 3p4 | [Ne] 3s 2 3p4 |
| 17 | Cl | 3 | 1s 2 2s 2 2p6 3s 2 3p5 | [Ne] 3s 2 3p5 |
| 18 | Ar | 3 | 1s 2 2s 2 2p6 3s 2 3p6 | [Ne] 3s 2 3p6 |
| 19 | K | 4 | 1s 2 2s 2 2p6 3s 2 3p6 4s 1 | [Ar] 4s 1 |
| 20 | Ca | 4 | 1s 2 2s 2 2p6 3s 2 3p6 4s 2 | [Ar] 4s 2 |
The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\). This provides the basis for a shorthand notation for electron configurations called the noble gas configuration, which atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of \(\left[ \ce{Ne} \right]\) for the \(1s^2 2s^2 2p^6\) part of the configuration. Sodium's noble gas configuration becomes \(\left[ \ce{Ne} \right] 3s^1\).
The electron filling diagram shown below in Figure \(\PageIndex{2}\) is useful in remembering the order for electrons to occupy shells and subshells. Although it is much easier to use the periodic table as a guide for electron filling as you will see in the next section.
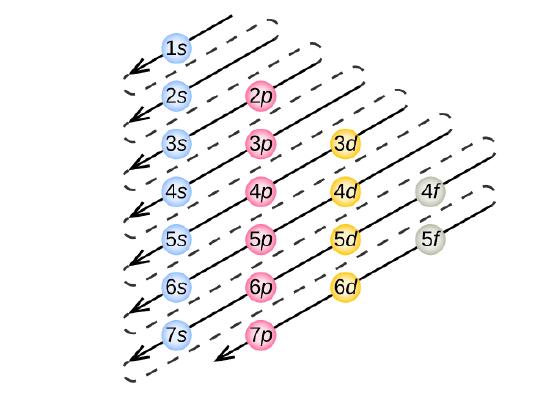
Using Figure \(\PageIndex{2}\) as your guide, write the electron configuration of a neutral phosphorus atom. The atomic number of P is 15.
Solution
A neutral phosphorus atom has 15 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 5 electrons. Of those 5 electrons, 2 can go into the 3s subshell, and the remaining 3 electrons can go into the 3p subshell. Thus, the electron configuration of neutral phosphorus atoms is 1s22s22p63s23p3.
Using Figure \(\PageIndex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. The atomic number of Cl is 17.
- Answer
-
A neutral chlorine atom has 17 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 7 electrons. Of those 7 electrons, 2 can go into the 3s subshell, and the remaining 5 electrons can go into the 3p subshell. Thus, the electron configuration of neutral chlorine atoms is 1s22s22p63s23p5.
Orbital Diagrams
An orbital diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital diagram, the individual orbitals are shown as squares and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its shell and sublevel. Electrons are indicated by arrows inside of the squares. An arrow pointing upwards indicates one spin direction, while a downward pointing arrow indicates the other direction. The orbital filling diagrams for hydrogen, helium, and lithium are shown in the figure below.
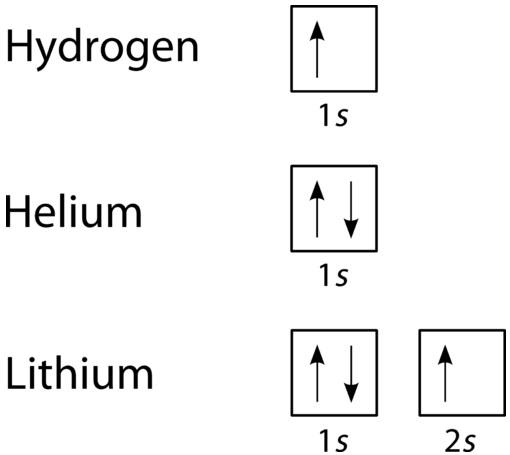
According to the Aufbau Principle, sublevels and orbitals are filled with electrons in order of increasing energy. Since the \(s\) sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium. The next element is lithium and necessitates the use of the next available sublevel, the \(2s\).
The orbital diagram for carbon is shown in Figure \(\PageIndex{10}\). There are two \(2p\) electrons for carbon and each occupies its own \(2p\) orbital (Hund's Rule).

Oxygen has four \(2p\) electrons. After each \(2p\) orbital has one electron in it, the fourth electron can be placed in the first \(2p\) orbital with a spin opposite that of the other electron in that orbital.
If you keep your papers in manila folders, you can pick up a folder and see how much it weighs. If you want to know how many different papers (articles, bank records, or whatever else you keep in a folder), you have to take everything out and count. A computer directory, on the other hand, tells you exactly how much you have in each file. We can get the same information on atoms. If we use an orbital filling diagram, we have to count arrows. When we look at electron configuration data, we simply add up the numbers.
Draw the orbital filling diagram for carbon and write its electron configuration.
Solution
Step 1: List the known quantities and plan the problem.
Known
- Atomic number of carbon, Z=6
Use the order of fill diagram to draw an orbital filling diagram with a total of six electrons. Follow Hund's rule. Write the electron configuration.
Step 2: Construct the diagram.
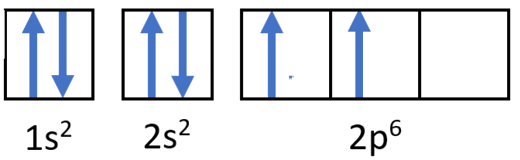
Orbital filling diagram for carbon.
Electron configuration 1s22s22p2
Step 3: Think about your result.
Following the 2s sublevel is the 2p, and p sublevels always consist of three orbitals. All three orbitals need to be drawn even if one or more is unoccupied. According to Hund's rule, the sixth electron enters the second of those p orbitals and has the same spin as the fifth electron.

