6.20: Periodic Trends - Ionic Radii
- Page ID
- 53711
How are peanuts sold?
Peanuts can be sold two ways. The bare peanut without the shell (brown inner portion of peanut) can be purchased in jars and packages for casual munching or for cooking. The size of the peanut in this situation is smaller than the peanut plus shell since the outer portion is missing. If we add the shell to the peanut, we have a larger size for the combination.
Electrons and protons are strongly attracted to one another. The strength of that attraction and the relative numbers of the two particles in a given atom or ion have a significant influence on the size of that species. When an atom loses one or more electrons, the resulting ion becomes smaller. If electrons are added to the atom, the ion becomes larger.
Ionic Radius
The ionic radius for an atom is measured in a crystal lattice, requiring a solid form for the compound. These radii will differ somewhat depending upon the technique used. Usually, x-ray crystallography is employed to determine the radius for an ion.
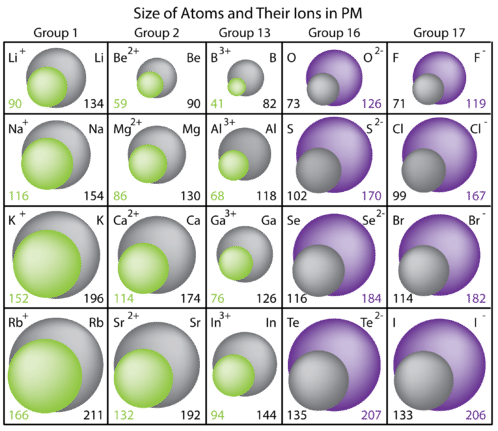
The removal of electrons always results in a cation that is considerably smaller than the parent atom. When the valence electron(s) are removed, the resulting ion has one fewer occupied principal energy level, so the electron cloud that remains is smaller. Another reason is that the remaining electrons are drawn closer to the nucleus because the protons now outnumber the electrons. One other factor is the number of electrons removed. The potassium atom has one electron removed to form the corresponding ion, while calcium loses two electrons.
The addition of electrons always results in an anion that is larger than the parent atom. When the electrons outnumber the protons, the overall attractive force that the protons have for the electrons is decreased. The electron cloud also spreads out because more electrons results in greater electron-electron repulsion. Notice that the group 16 ions are larger than the group 17 ions. The group 16 elements each add two electrons, while the group 17 elements add one electron per atom, to form the anions.
Summary
- Ionic radius is determined by measuring the atom in a crystal lattice.
- Removal of electrons results in an ion that is smaller than the parent element.
- Addition of electrons results in an ion that is larger than the parent atom.
Review
- How are ionic radii measured?
- Explain why the radius of the rubidium ion is smaller than the radius of the rubidium atom.
- Explain why the radius of the tellurium ion is larger than the radius of the tellurium atom.
- Why is the oxygen anion larger than the fluoride anion?
- Why is the sodium cation larger than the magnesium cation?

