3.2: Organometallic Compounds of Boron and Aluminium
- Page ID
- 172732
Learning Objectives
In this lecture you will learn the following
- Preparation and reactivity of organoboron and organo aluminium compounds.
- Influence of Lewis acidity on structural features
Organoboron Compounds
BMe3 is colorless, gaseous ( b.p. -22 °C), and is monomeric. It is pyrophoric but not rapidly hydrolyzed by water.
Alkylboranes can be synthesized by metathesis between BX3 and organometallic compounds of metals with low electronegativity, such as RMgX or AlR3.
\[\ce{BF3 + 3CH3MgBr -> B(CH3)3 + 3MgBrF (solvent\: used: \: dibutyl\: ether)} \nonumber \]
Why dibutyl ether as a solvent: Has much lower vapor pressure than BMe3 and as a result the separation by trap-to-trap distillation on a vacuum line is easy.
Also, there is a very weak association between BMe3 and OBu2(Me3B:OBu2).
Although, trialkyl- and triarylboron compounds are mild Lewis acids, strong carbanion reagents lead to anions of the type [BR4]-.
Example, Na[BPh]4: The bulky anion hydrolyses very slowly in neutral or basic water and is useful for the preparation of large positive cations.
\[\ce{Na[BPh]4 + K+ -> K[BPh]4} \nonumber \]
K[BPh]4 is insoluble, used for the gravimetric estimation (determination) of potassium, an example of the low solubility of large-cation and large-anion salts in water/
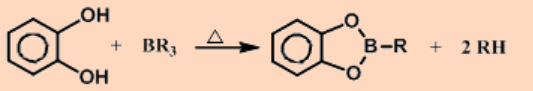
Organohaloboron compounds are more reactive than simple trialkylboron compounds.
Preparation:
\[\ce{2 BCl3 + 6AlR3 -> 3R2BCl + 6AlR2Cl (metathesis)} \nonumber \]
\[\ce{2 BCl3 + BMe3 ->[(diborane)] 3BMeCl2 (redistribution\:reaction)} \nonumber \]
Reactions: (Protolysis reactions with ROH, R2NH and other reagents)
\[\ce{3BMeCl2 + 2HNR2 -> BMe2(NR2) + [R2NH2]Cl} \nonumber \]
\[\ce{BMe2Cl + Li(C4H9) -> BMe2(C4H9) + LiCl} \nonumber \]
Organoaluminium compounds
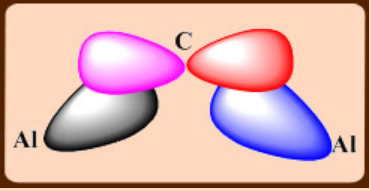
With less bulky alkyl groups, dimerization occurs and one of the distinguishing features of alkyl bridge is the small Al-C-Al angle, which is ~ 75°.
The 3c,2e bonds are very weak and tend to dissociate in the pure liquid which increases with increase in the bulkiness of the alkyl group.
\[\ce{Al2(CH3)6 <=> 2Al(CH3)3} \nonumber \] K= 1.52 x 10-8
\[\ce{Al2(C4H9)6 <=> 2Al(C4H9)3} \nonumber \] K= 2.3 x 10-4
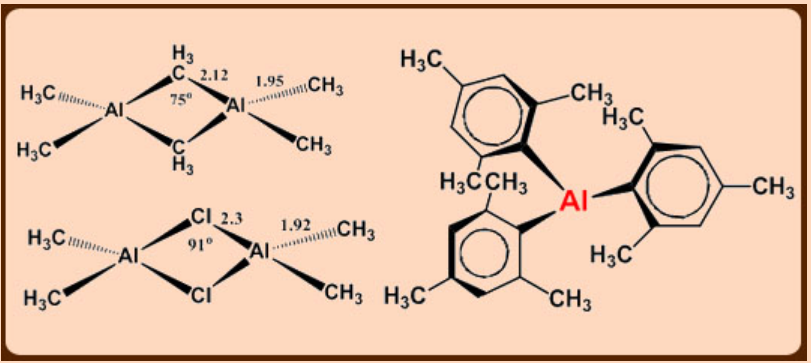
Perpendicular orientation of pheynl groups in Al2Ph6
Triphenylaluminium exists as a dimer with bridging η1-phenyl groups lying in a plane perpendicular to the line joining the two Al atoms.
This structure is favored partly on steric grounds and partly by supplementation of the Al-C-Al bond by electron donation from the phenyl π-orbitals to the Al atoms.
Tendency for bridging: X > Ph > alkyl
3c,2e bonds formed by a symmetric combination of Al and C orbitals
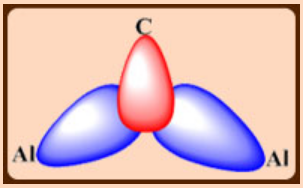
An additional interaction between the pπ orbital on C and an antisymmetric combination of Al orbitals.
Synthesis
Very useful as alkene polymerization catalysts and chemical intermediates.
Expensive carbanion reagents for the replacement of halogens organic groups by metathesis.
Laboratory scale preparations involves: \[\ce{2Al + 3Hg(CH3)2 -> Al2(CH3)6 + 3Hg} \nonumber \]
Commercial method: \[\ce{2Al + CH3Cl -> Al2Cl2(CH3)4} \nonumber \]
\[\ce{Al2Cl2(CH3)4 + 6Na -> Al2(CH3)6 + 2Al + 6NaCl} \nonumber \]
Commercial method for ethylaluminium and higher homologs: \[\ce{2Al + 3H2 + 6RHC=CH2 ->[60-110°C][110-200atm] 2Al2(CH2CH2R)6} \nonumber \]
The reaction probably proceeds by the formation of a surface Al—H species that adds across the double bond of the alkene in a hydrometallation reaction.
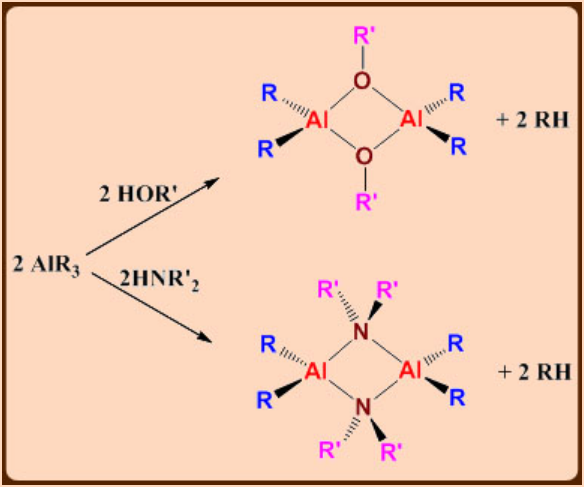
Reactions:
Alkylaluminum compounds are mild Lewis acids and form complexes with ethers, amines and anions. When heated, often β-hydrogen elimination is responsible for the decomposition of ethyl and higher alkylaluminium compounds. E.g. Al(iC4H9)3
Tendency towards bridging structure is: PR2-> X -> H -> Ph-> R-.
Problems:
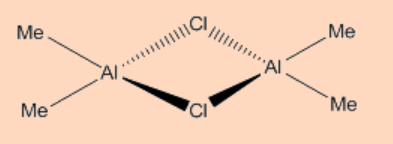
1. Propose a structure for Al2(Me)4Cl2.
Solution:
Similar to diborane:
2. For these compounds (H3Si)2O and (CH3CH2)2O, which do you expect to have the lower force constant, Si-O-Si bending or C-O-C bending?
Solution:
Lower force constant for Si-O-Si bending.
3. Explain how the difference in reactivity between Al-C and Si-C bonds with O-H groups leads to the choice of different strategies for the synthesis of aluminum and silicon alkoxides.
Solution
\[\ce{Al2Me6 + 6MeOH -> 2Al(OMe)3 + 6CH4} \nonumber \]
For reaction of Al2Me6 with alcohols, see the text book by Shriver and Atkins.
Tetramethylsilane does not react with methyl alcohol. Therefore, the appropriate reagent is tetrachlorosilane and the reaction is:
\[\ce{SiCl3 + 4MeOH -> Si(OMe)4 + 4HCl} \nonumber \]
4. Compare formulas of the most stable hydrogen compounds of germanium and arsenic with those of their methyl compounds. Can the differences be explained in terms of the relative electronegativities of C and H?
Solution
GeH4, GeR4; AsH3, AsR3
The stability of hydrides and alkyls are very similar for each element. This may due to similar H and C electronegativity.
5. To buy from a chemical company, the price of trimethylaluminum is higher than that of triethylaluminum. Is it due to the methods of synthesis? Rationalize the price difference.
Solution:
Triethylaluminum can be made in larger quantities by direct reaction of aluminum, hydrogen gas and ethane gas which is a cheaper method.
\[\ce{2Al + 3H2 + 6RHC=CH2 ->[60-110°C][110-200atm] 2Al2(CH2CH2R)6} \nonumber \]
Preparation of trimethyaluminum involves a more expensive route such as MeCl and aluminum to form Al2Me4Cl2 followed by treatment with sodium metal. The sodium metal and MeCl are not cheap as compared to ethane and hydrogen gases.
\[\ce{2Al + CH3Cl -> Al2Cl2(CH3)4} \nonumber \]
\[\ce{Al2Cl2(CH3)4 + 6Na -> Al2(CH3)6 + 2Al + 6NaCl} \nonumber \]


