4.6: More on Electronic Effects
- Page ID
- 189773
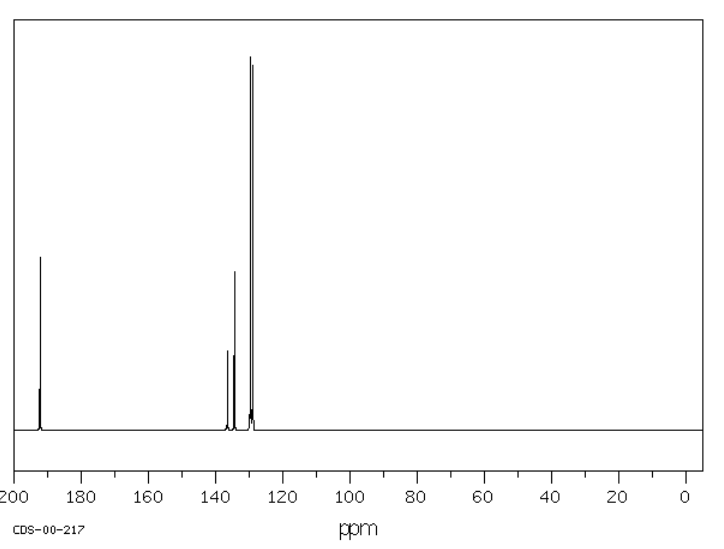
Methoxybenzene or anisole has six carbons, but only four peaks in the spectrum because of symmetry. These peaks are all above 100 ppm, but some peaks are as far downfield as 160 ppm.
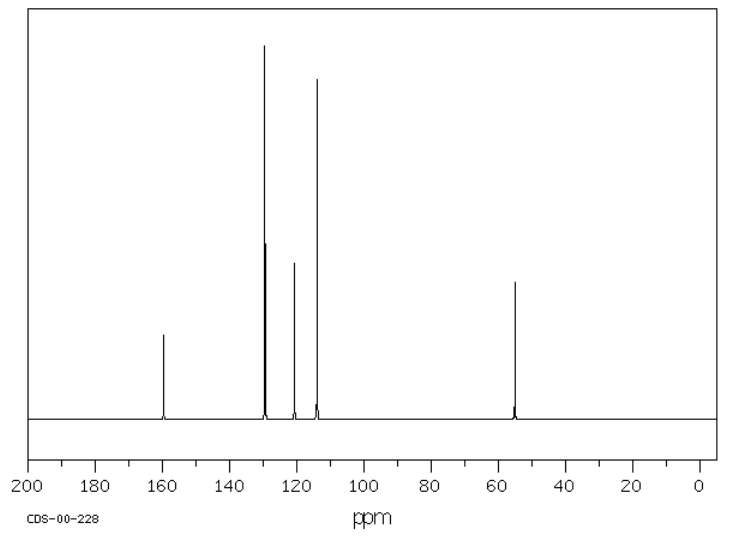
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 15 August 2008)
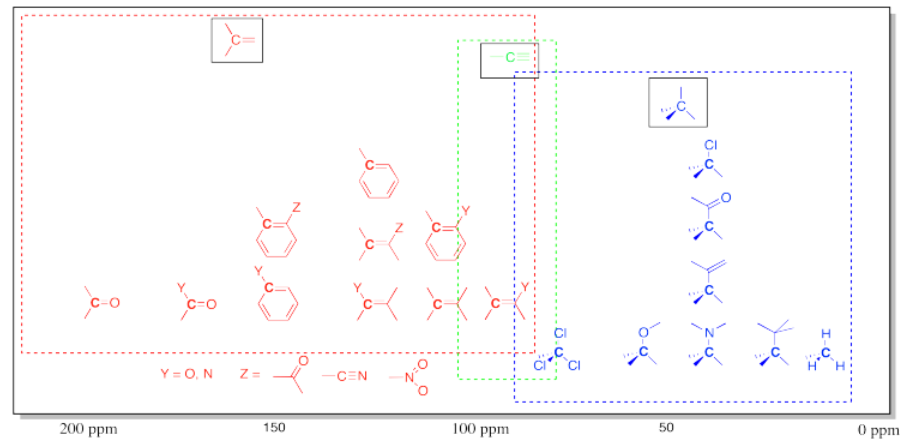
- usually, a trigonal planar carbon shows up in the downfield half of the spectrum.
- the region from about 100 to 200 ppm can be thought of as the sp2 window.
- an electronegative atom moves a peak further downfield within the sp2 window.
Benzaldehyde has peaks between 130 and 140 ppm, as well as one near 190 ppm. Just as in the sp3 region of the spectrum, when a carbon is attached to an electronegative element, it moves further downfield, and since the carbonyl (or C=O) carbon in the aldehyde has two bonds to oxygen, it shows up considerably downfield. The carbonyl carbon in some ketones can show up as far as 210 ppm.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 15 August 2008)
- Carbonyl carbons may show up even further downfield than 200 ppm.
- Carbonyl carbons have a great deal of positive charge and low electron density.
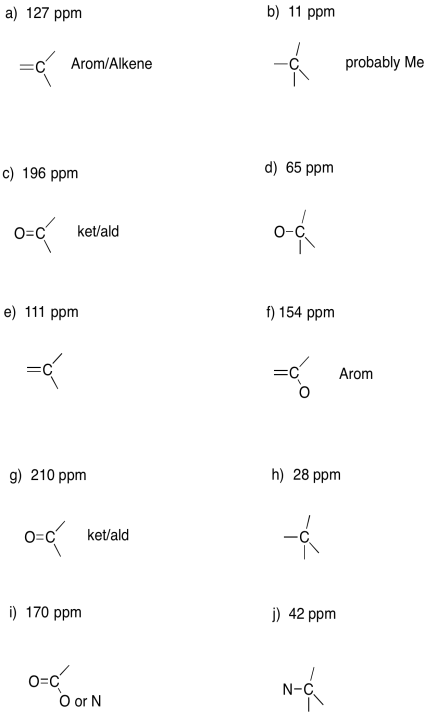
Suggests possible assignments for the following chemical shifts in a 13C NMR spectrum.
a) 127 ppm b) 11 ppm c) 196 ppm d) 65 ppm e) 111 ppm
f) 154 ppm g) 210 ppm h) 28 ppm i) 170 ppm j) 42 ppm
- Answer
-
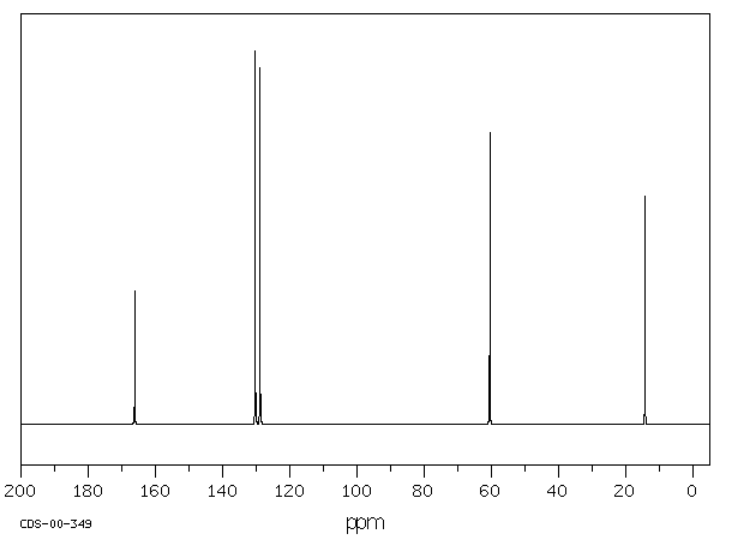
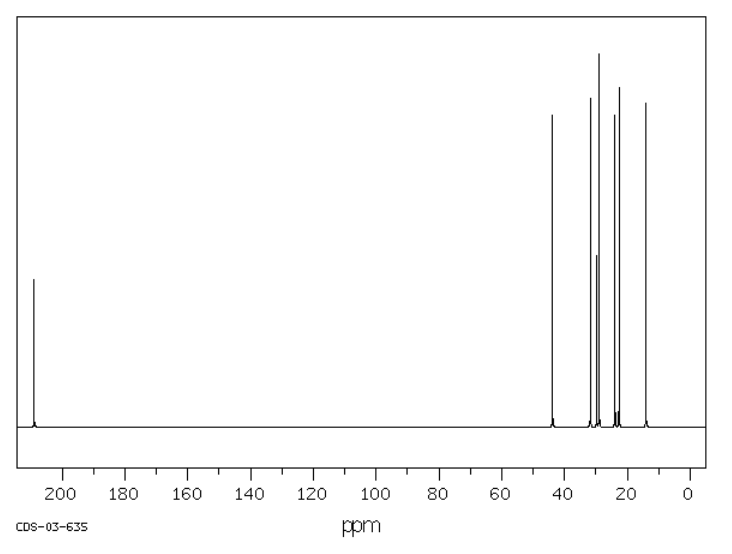
Suggest possible structures for the following spectra.
Source: SDBSWeb : riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 19 August 2008)
a)
b)
c)
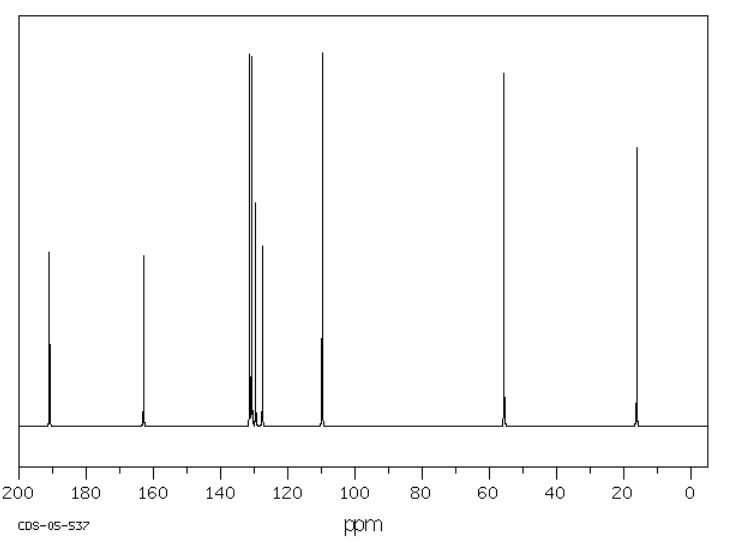
- Answer
-
There are subtle effects of electronegativity in saturated hydrocarbons like hexane. Carbons in different positions in those compounds show up at different shifts. In general, a methyl group (CH3) will show up farther upfield than a methylene group (CH2), which will in turn show up further upfield from a methyne group (CH). This trend is related to the difference in electronegativity between a carbon and a hydrogen. Carbon is slightly more electronegative than a hydrogen. As a result, a carbon atom that is bonded to a number of hydrogen atoms has a very slight negative charge. That means it absorbs further upfield. A carbon that is bonded to a number of carbons is more neutral, is not quite so shielded, and shows up a little more downfield.
Unless there are bigger electronegative effects due to heteroatoms such as oxygen,
- methyl (CH3) groups show up furthest upfield.
- methylene (CH2) groups show up next furthest upfield.
- methyne (CH) groups show up furthest downfield.
We can use NMR spectroscopy as a diagnostic tool to determine the structure of a compound. 13C NMR spectroscopy is useful in highlighting whether there are any double bonds in a molecule, whether there are any heteroatoms such as oxygen, and how many different kinds of carbons there are. That last point isn't the same as the number of carbons, but is related to the symmetry of the molecule.
Table \(\PageIndex{1}\): Approximate chemical shifts in 13C NMR spectroscopy.