10.3: Respiratory Tract
- Page ID
- 317860
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Respiratory Tract
Many environmental and occupational agents as well as some pharmaceuticals enter the respiratory tract through inhalation. Absorption can occur at any place within the upper respiratory tract. However, the amount of a particular xenobiotic that can be absorbed at a specific location depends highly on its physical form and solubility.
There are three basic regions to the respiratory tract:
- Nasopharyngeal region
- Tracheobronchial region
- Pulmonary region
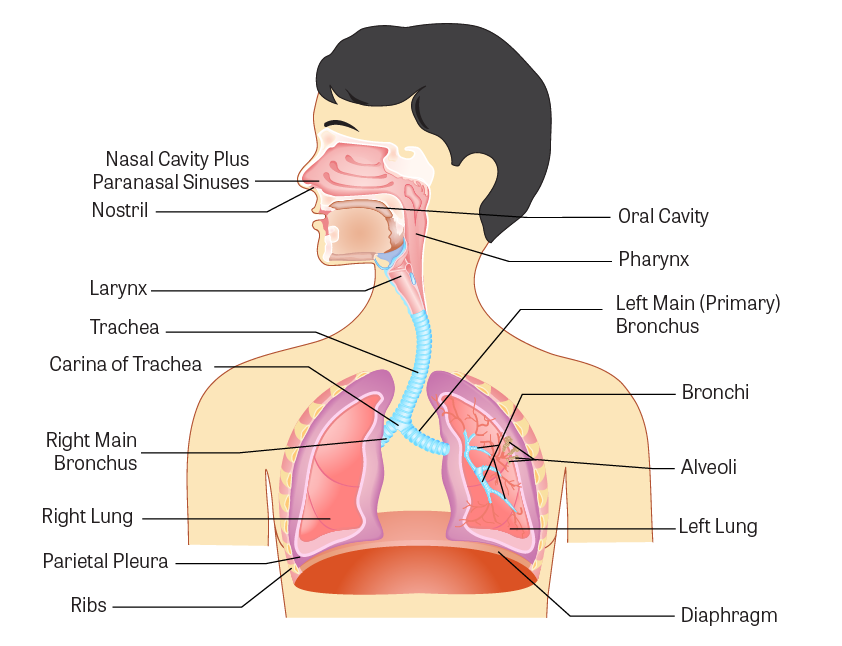
Figure \(\PageIndex{1}\). Anatomy of the respiratory tract
(Image Source: adapted from iStock Photos, ©)
Mucociliary Escalator
The mucociliary escalator covers most of the bronchi, bronchioles, and nose. It contains mucus-producing goblet cells and ciliated epithelium. The movements of the cilia push it and anything in it such as inhaled particles or microorganisms up and out into the throat, which can either get swallowed or removed through the mouth.
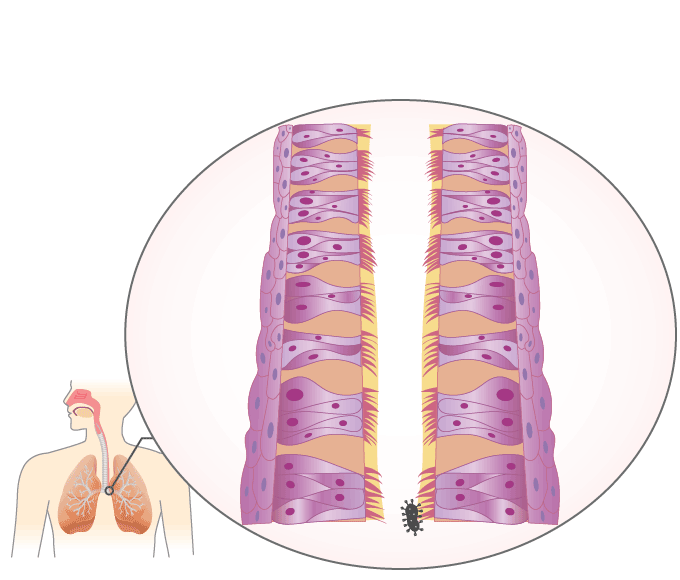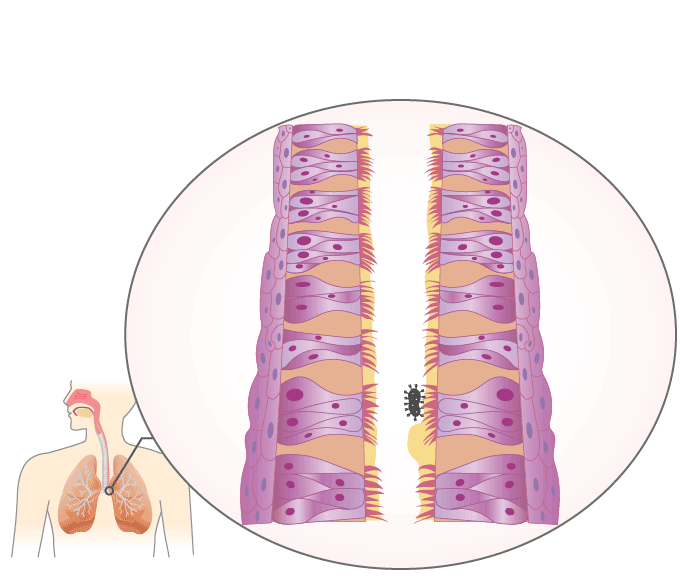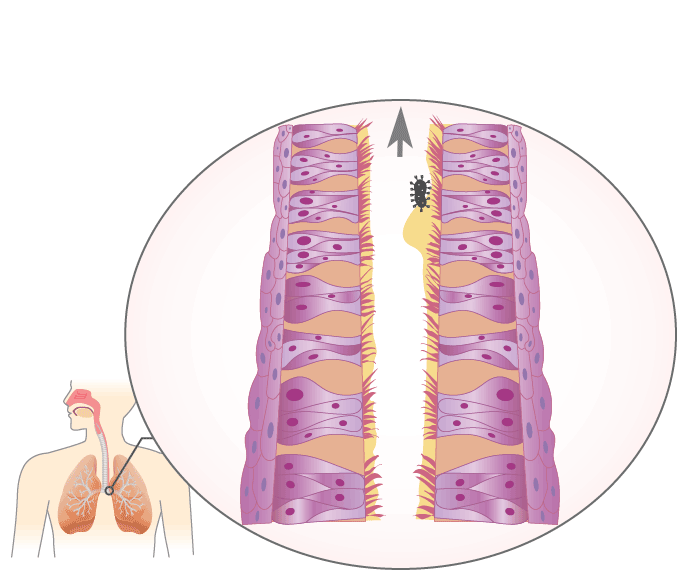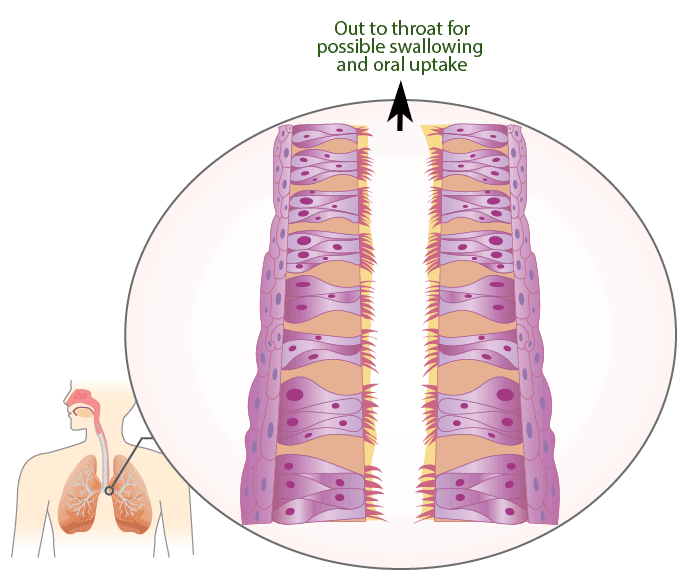
Animation \(\PageIndex{1}\). The mucociliary escalator provides a barrier against infection
Pulmonary Region
By far, the most important site for absorption is the pulmonary region consisting of the very small airways called bronchioles and the alveolar sacs of the lung.
The alveolar region has a very large surface area, about 50 times that of the skin. In addition, the alveoli consist of only a single layer of cells with very thin membranes that separate the inhaled air from the blood stream. Oxygen, carbon dioxide, and other gases readily pass through this membrane. Gases and particles, which are water-soluble (and thus blood-soluble), are absorbed more efficiently from the lung alveoli compared to their absorption via the gastrointestinal tract or through the skin. Water-soluble gases and liquid aerosols can pass through the alveolar cell membrane by simple passive diffusion.

Figure \(\PageIndex{2}\). Detailed view of alveoli and bronchioles
(Image Source: adapted from iStock Photos, ©)
Impact of Physical Form on Absorption
In addition to solubility, the ability to be absorbed depends highly on the physical form of the agent (that is, whether the agent is a gas/vapor or a particle). The physical form determines the extent of its penetration into the deep lung.
Gases and Vapors
A gas or vapor can be inhaled deep into the lung and if it has high solubility in the blood, it is almost completely absorbed in one respiration (a single breath). Absorption through the alveolar membrane is by passive diffusion, following the concentration gradient. As the agent dissolves in the circulating blood, it leaves the lung and a large amount of gas or vapor can be absorbed and enter the body.
Blood-soluble gases or vapors can often be exhaled instead of absorbed right away. For blood-soluble gases, the equilibrium between the concentration of the agent in the inhaled air and that in the blood is difficult to achieve. Inhaled gases or vapors, which have poor solubility in the blood, have a limited capacity for absorption. The main reason for this is that the blood can become quickly saturated. Once saturated, blood will not be able to accept the gas and it will remain in the inhaled air and then get exhaled. One way that the amount of gas absorbed could increase is if the rate and depth of breathing were increased (this concept is known as ventilation limitation). More specifically, the amount of gas absorbed could be increased if the rate of blood supply to the lung were increased by flow limitation.
In contrast, insoluble gases or vapors can be absorbed into the body by the lungs before getting exhaled (for example, nitrogen dioxide and carbon monoxide). This is because the equilibrium between the inhaled air and the blood is reached more quickly for relatively insoluble gases than for soluble gases.
Airborne Particles
The absorption of airborne particles is usually quite different from that of gases or vapors. The absorption of solid particles, regardless of solubility, depends upon particle size:
- Large particles (>5 μM) are generally deposited in the nasopharyngeal (head airways region) region with little absorption.
- Particles 2-5 μM can penetrate into the tracheobronchial region.
- Very small particles (<1 μM) are able to penetrate deep into the alveolar sacs where they can deposit and be absorbed.
Differences in Absorption Among Regions of the Respiratory Tract
Nasopharyngeal Region
Minimal absorption takes place in the nasopharyngeal region due to the cell thickness of the mucosa and the rapid movement of gases and particles through the region.
Tracheobronchial Region
Relatively soluble gases can quickly enter the blood stream. Most deposited particles are moved back up to the mouth where they are swallowed.
Pulmonary Region
Absorption in the alveoli of the pulmonary region is quite efficient compared to other areas of the respiratory tract. Relatively soluble materials (gases or particles) are quickly absorbed into systemic circulation. Pulmonary macrophages exist on the surface of the alveoli. They are not fixed and not a part of the alveolar wall. They can engulf particles just as they engulf and kill microorganisms. These alveolar macrophages can scavenge and clear some insoluble particle into the lymphatic system.

Figure \(\PageIndex{3}\). Alveoli
(Image Source: iStock Photos, ©
Some other particles may remain in the alveoli indefinitely. For example:
- Coal dust and asbestos fibers may lead to black lung or asbestosis, respectively.
- Carbon nanotubes (CNT), tiny tube-shaped structures smaller than a human hair, have been found in the lungs long after exposure. CNT are used in materials like polymers and anti-static packaging for their electric, magnetic, and mechanical properties. Studies of what happens to different forms of single-walled and multi-walled carbon nanotubes (CNT) found that pristine CNT could remain in the lung for months or even years after pulmonary deposition. However, some CNT can move to the gastrointestinal (GI) tract via the mucocilary escalator where, if swallowed, there appears to be no uptake of CNT from the GI tract (with a possible exception of the smallest functionalized single-walled CNT). In addition, under some experimental conditions in animals, some carbon nanotubes moved from the alveolar space to the nearby pulmonary region including lymph nodes, subpleura and pleura, and smaller amounts went to distal organs including the liver, spleen, and bone marrow.
Factors Affecting the Toxicity of Inhaled Materials
The nature of toxicity of inhaled materials depends on whether the material is absorbed or remains within the alveoli and small bronchioles. If the agent is absorbed and is lipid soluble, it can rapidly distribute throughout the body, passing through the cell membranes of various organs or into fat depots. Lipid-soluble substances take a longer time to reach equilibrium than water soluble substances. Chloroform and ether are examples of lipid-soluble substances with high blood solubility.
Non-absorbed foreign material can also cause severe toxic reactions within the respiratory system. These reactions may take the form of chronic bronchitis, alveolar breakdown (emphysema), fibrotic lung disease, and even lung cancer. In some cases, the toxic particles can kill the alveolar macrophages, which results in lowering the body's respiratory defense mechanism.
Pharmaceuticals Targeted to the Respiratory Tract
Inhaled drug delivery devices can be very effective and safe for getting active agents directly to their site of action. Inhalation is used to deliver locally acting drugs to treat respiratory conditions, including asthma, chronic obstructive pulmonary disease (COPD), and airway infections. Advantages of targeted delivery to the lungs include a more rapid onset of action and an increased therapeutic effect. Depending on the drug inhaled, there can be reduced systemic side effects since a lower dose can deliver the required local concentration.
Toxicogenomics and Toxicity Testing
Toxicogenomics applies genomics concepts and technologies to study gene and protein activities within a type of tissue or type of cell in response to a chemical exposure. Toxicogenomics helps in the understanding of what genes and proteins interact with a chemical, and what diseases are associated with various genes, proteins, and chemicals. This example used toxicogenomics to evaluate the response of the respiratory tract to one type of inhaled material:
For example, titanium dioxide nanoparticles (TiO2NPs) induce lung inflammation in experimental animals and one study included a comprehensive toxicogenomic analysis of lung responses in mice exposed to six individual TiO2NPs exhibiting different sizes, crystalline structure, and surface modifications. The goal was to investigate whether the mechanisms leading to TiO2NP-induced lung inflammation are property specific. The results suggest that the severity of lung inflammation is property specific; however, the underlying mechanisms (genes and pathways perturbed) leading to inflammation were the same for all particle types. While the particle size clearly influenced the overall acute lung responses, a combination of small size, crystalline structure, and hydrophilic surface contributed to the long-term pathological effects observed at the highest dose.
1) An inhaled material will most likely be absorbed into the body if it has the following characteristics:
a) High lipid solubility and poorly ionized
b) Large particle size and low water solubility
c) High water solubility and small particle size
- Answer
-
High water solubility and small particle size - This is the correct answer.
In contrast to absorption via the gastrointestinal tract or through the skin, gases and particles, which are water-soluble (and thus blood soluble), will be absorbed more efficiently from the lung alveoli. Very small particles (<1 μM) are able to penetrate deep into the alveolar sacs where they can deposit and be absorbed.
2) Particles of size 2-5 μM are most likely to settle out in which location of the respiratory tract?
a) Nasopharyngeal region
b) Tracheobronchial region
c) Pulmonary region
- Answer
-
Tracheobronchial region - This is the correct answer.
Particles 2-5 µM can penetrate into the tracheobronchial region. Very small particles (<1 μM) are able to penetrate deep into the alveolar sacs where they can deposit and be absorbed.


