16.6: Protecting Water, Food, and Air
- Page ID
- 285403
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The tragedy that arguably illustrates most vividly the potential of chemicals to cause major loss of life was the accidental release of methyl isocyanate from a chemical manufacturing operation in Bhopal, India. This disaster occurred during the night of December 2/3, 1984, exposing many victims as they slept in their homes. A total of about 40 tons of this chemical was released during the incident exposing thousands of residents in the surrounding area. Of those exposed, more than 3000 died, primarily from pulmonary edema (fluid accumulation in the lung). Immunological, neurological, ophthalmic (eye), and hematological effects were also observed. Because of its high vapor pressure and toxicity to multiple organs,
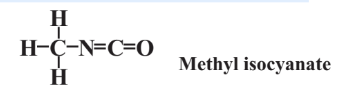
methyl isocyanate is the most toxic of the isocyanates. An interesting aspect of methyl isocyanate toxicity is its ability to cross cell membranes and reach organs far from the site of exposure, despite its very high reactivity.
It is unlikely that methyl isocyanate or of any other substance with similar toxicity could be obtained and delivered to potential victims in large enough quantities and with sufficiently effective delivery to cause widespread death and illness in a deliberate attack. Nevertheless, the magnitude of the Bhopal catastrophe vividly illustrates the potential of toxic substances to cause harm and the incident serves as a grim reminder of the potential of toxic chemicals, especially those that may be airborne, to be used in attacks. And the Bhopal incident, which occurred as the result of a tragic accident, serves as a grim warning of the potential of terrorist attacks upon chemical plants to cause widespread harm.
Potential Chemical Agents
Although an unlikely “weapon of mass destruction,” carbon monoxide, CO, can certainly cause fatal poisonings. It has killed thousands of people accidentally and through suicide. Given the fact that carbon monoxide is odorless and provides essentially no warning of exposure, it should be regarded as a potential weapon. To understand the action of carbon monoxide, it is important to realize that oxygen in blood is carried from the lungs to tissue by hemoglobin, a large-molecule protein in red blood cells that contains iron(II) (the Fe2+ ion) bound with nitrogen. This functionality exchanges oxygen
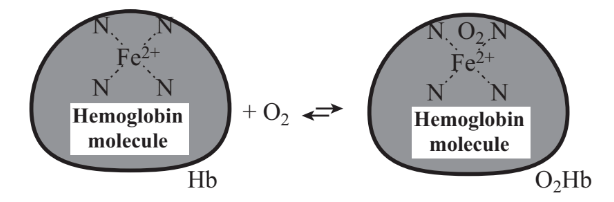
converting hemoglobin, Hb, into oxyhemoglobin, O2Hb, which carries oxygen to tissues where it is released to be used for metabolic processes. When carbon monoxide is present in inhaled air, the oxygen bound to hemoglobin is displaced,
\[\ce{O2Hb + CO <=> COHb + O2}\]
producing carboxyhemoglobin, COHb. This species is not only useless for carrying oxygen, it is much more stable than oxyhemoglobin, O2Hb, so that a relatively low concentration of carbon monoxide will convert enough of the hemoglobin to carboxyhemoglobin to cause a serious oxygen deficiency. Rapid death ensues from inhalation of air containing 1,000 parts per million (ppm) carbon monoxide, and unconsciousness results from inhaling 250 ppm CO. Dizziness, headache, and fatigue result from inhalation of 100 ppm CO and levels as low as 10 ppm can impair visual perception and judgment.
Chlorine (Cl2) gas could potentially be used in terrorist attacks because of its wide availability for water disinfection and other uses. Illustrative of this potential is the fact that chlorine was the first substance used as a military “poison gas” in World War I. Chlorine is a strong oxidizer that reacts with water, including water in tissue, to produce an oxidizing, acidic solution that is especially damaging to respiratory (lung) tissue. Air containing only 10–20 ppm chlorine causes acute discomfort to the respiratory tract and brief exposure to 1,000 ppm of Cl2 can be fatal. Because of its widespread use and transport as the liquid in railway tank cars, chlorine is regarded as having a high potential as a terrorist weapon.
Hydrogen cyanide, HCN, is a potentially devastating gaseous pollutant. It has been used to carry out death sentences in gas chambers, causing death very rapidly when inhaled. Another toxic form of cyanide is cyanide ion, CN-, in salts such as KCN. Only about 60 mg of KCN will kill a human. Glass ampules containing KCN or liquid HCN were used by some doomed Nazi leaders to commit suicide near the end of World War II. There is concern that potassium cyanide or other soluble cyanide salts may be put into water supplies as toxic agents.
The metabolic action of cyanide depends upon its strong binding for iron in the +3 oxidation state. In the essential utilization of molecular oxygen in the body (the respiration process called oxidative phosphorylation) iron cycles between iron(III) in ferricytochrome oxidase enzyme andiron(II) in the chemically reduced form, ferrous cytochrome oxidase. By stabilizing ferricytochrome oxidase, cyanide stops this cycle, preventing utilization of oxygen and causing metabolic processes to cease. It is interesting to note that an antidote to cyanide poisoning — in those rare instances where the victim survives long enough for antidotes to be administered — is to have the victim inhale a volatile nitrite ester. The nitrite converts a fraction of the iron(II) in hemoglobin to iron(III), generating methemoglobin. This form of hemoglobin cannot carry oxygen, but it can bind with cyanide, preventing it from tying up ferricytochrome oxidase enzyme.
Hydrogen sulfide, H2S, is a colorless gas with a foul, rotten-egg odor that is as toxic as hydrogen cyanide and may kill even more rapidly. Inhalation of 1000 ppm hydrogen sulfide causes rapid death from respiratory system paralysis. Nonfatal doses of this gas can cause excitement due to damage to the central nervous system; headache and dizziness may be symptoms of exposure.
Military Poisons and Nerve Gas Agents
Beginning with the use of toxic chlorine gas in World War I, nations have developed a variety of diabolical toxic agents to disable and kill opposing troops in war. One such agent is sulfur mustard, a class of chemical compounds, the most common of which is bis(2-chloroethyl)sulfide
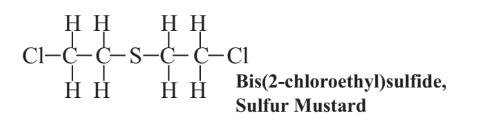
The vapors of this substance penetrate rapidly and deeply into tissue causing tissue damage and destruction well below the point of entry. Because of its penetrating ability, efforts to remove sulfur mustard from exposed tissue are futile after about 30 minutes. Sulfur mustard is classified as a vesicant (“blistering gas”) producing severely inflamed lesions that are susceptible to infection. Such lesions in the lungs are likely to be fatal. (An internet search for pictures of sulfur mustard lesions will bring up some horrifying images of the wounds that this material can inflict.) Sulfur mustard causes mutations because it forms a reactive intermediate that binds with DNA and is thought to be a primary carcinogen that does not require metabolic activation to produce cancer.
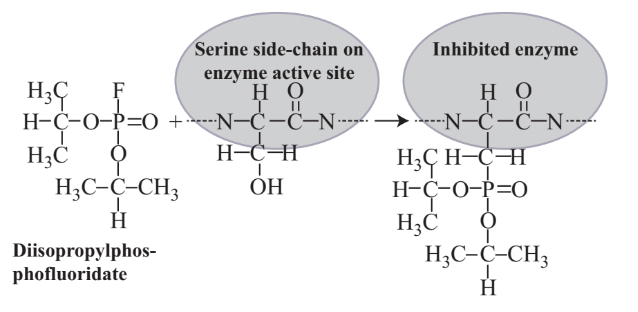
The chemical agents of greatest concern for their potential use in terrorist attacks are the organophosphorus “nerve gases.” The first of these deadly agents was reported in 1937 by Gerhard Schrader of the German concern Farbenfabriken Bayer AG. Work continued on these compounds in Germany during World War II and by other nations after the war and during the cold war between Western and Communist bloc nations until around 1990. The possibility that Iraq possessed large stores of military nerve gas “weapons of mass destruction” was part of the rationale for the U.S./Iraq war in 2003. Among the common nerve gases are Sarin, Soman, Tabun, CMPF, VX, and diisopropylphosphofluoridate (fluorodiisopropyl phosphate)Structural formulas of three of these compounds are shown in Figure 16.4.
Sarin is perhaps the best known organophosphorus military poison because of its use in a 1995 attack by a terrorist group on the Tokyo subway system that killed several people and caused illness in a number of others. It is estimated that a dose of only about 0.01 milligrams of Sarin per kilogram of body mass is fatal; absorption of a single drop of liquid Sarin through the skin can kill a human. Sarin and other organophosphate military poisons act on the nervous
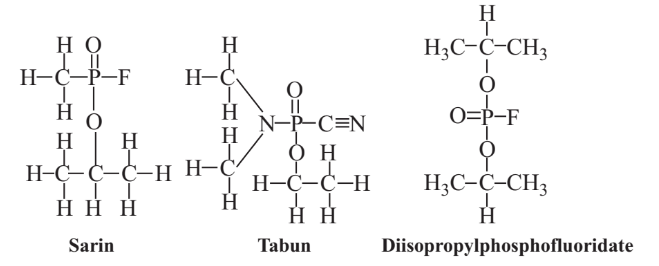
system by binding with and inhibiting acetylcholinesterase enzyme, which is required to hydrolyze acetylcholine and stop nerve impulses once their function has been completed. The failure of the hydrolysis of acetylcholinesterase typically causes failure of the respiratory system. The binding of diisopropylphosphofluoridate to a serine side-chain on the active site of acetylcholinesterase enzyme is shown by the reaction below:
 \[ \: \]
\[ \: \]
Biotoxins
Some of the most toxic substances known are produced by organisms and some of these have been used to attack humans.Clostridium botulinum bacteria growing in the absence of oxygen produce botulinum toxin, which has killed many people who have eaten improperly canned food (heating to 80–100 ̊C for a sufficient time deactivates the toxin). Consisting of several proteins, botulinum toxin binds irreversibly to nerve terminals preventing the release of acetylcholine, an enzyme required for proper nerve function. Neurologic symptoms are followed by paralysis of the respiratory muscles and death. From the LD50 of about 1×10-5 mg/kg for botulinum toxin shown in Figure 16.1, it may be inferred that a 70 kg person could die from absorbing 70 kg 1×10-5mg/kg = 0.0007 mg of botulinum toxin, or only about 1 microgram of this extraordinarily deadly substance. A simple calculation would show that literally millions of people could be killed by the amount of this toxin that could be carried in a terrorist’s pocket if some efficient means could be found to deliver it.
Another highly toxic natural product is ricin, a very stable proteinaceous material extracted from castor beans (Ricinus communis). Only about 1/2 milligram of ricin (about the size of a pinhead) can be fatal when injected resulting in failure of kidneys, liver, and spleen along with massive blood loss from the digestive tract. Ricin gained notoriety in the 1978 assassination in London of the Bulgarian writer and journalist Georgi Markov injected with ricin from the tip of an umbrella. Although it is mentioned as a potential terrorist tool, ricin has its greatest toxicity by injection, which tends to limit its use as a weapon.


