15.2: What is Energy?
- Page ID
- 285390
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Energy is the ability to move matter around, that is, to do work. The movement of atoms and molecules is also a form of energy called heat. The energy contained in a moving mass of matter is kinetic energy. For example, energy collected from sunlight during the day can be accumulated in rapidly rotating spinning flywheels, then used at night when solar energy is not available. Water pumped into an elevated reservoir is an example of potential energy that can be run through a hydroelectric turbine to generate electricity as needed.
Chemical energy is a form of potential energy stored in the bonds of molecules. This energy can be released during chemical reactions, usually as heat but sometimes as electrical or light energy, as bonds are broken and new bonds are formed.
A crawler tractor equipped with a bulldozer for earth-moving illustrates the definition of energy and several forms of energy (Figure 15.1). Chemical energy in the form of petroleum hydrocarbons is used to fuel the tractor’s diesel engine. In the engine the hydrocarbons combine with oxygen from air,
\[\ce{2C16H34 + 49O2 \rightarrow 32CO2 + 34 H2O + heat energy}\]
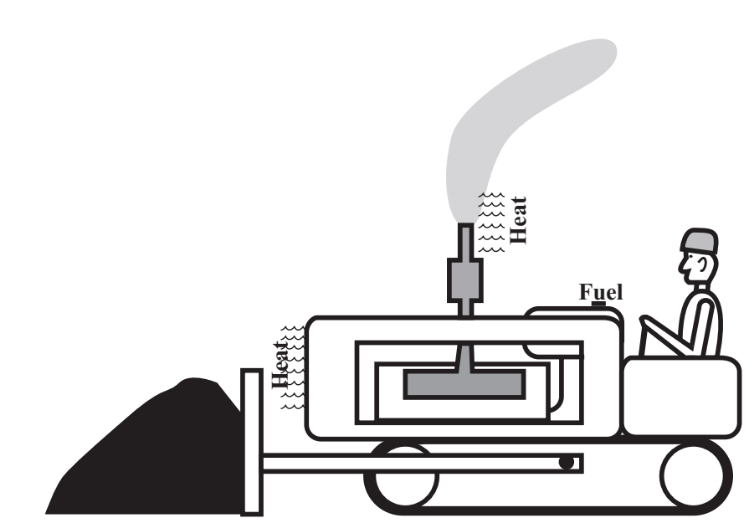
to produce heat energy. As the hot gases in the engine’s cylinders push the pistons down, some of this heat energy is converted to mechanical energy, which is transferred by the engine crankshaft, gears, axle, and tracks to propel the tractor forward. A blade or other implement attached to the tractor moves soil.
The energy released in a chemical reaction results from the difference in energies between the bonds in the reactants and the energy of the bonds in the products. An example calculation is shown for the combustion of a mole of methane in Chapter 5, Section 9.5.
Energy Units and Thermodynamics
The standard unit of energy is the joule, abbreviated J. A total of 4.184 J of heat energy will raise the temperature of 1 g of liquid water by 1 ̊C. This amount of heat is equal to 1 calorie of energy (1 cal = 4.184 J), the unit of energy formerly used in scientific work. A joule is a small unit, and the kilojoule, kJ, equal to 1000 J is widely used in describing chemical processes. The“calorie” commonly used to express the energy value of food (and its potential to produce fat) is actually a kilocalorie, kcal, equal to 1000 cal.
Power refers to energy generated, transmitted, or used per unit time. The unit of power is the watt equal to an energy flux of 1 joule per second (J s-1). A compact fluorescent light bulb adequate to illuminate a desk area might have a rating of 21 watts. A large powerplant may put out electricity at a power level of 1000 megawatts (mw, where one mw is equal to 1 million watts). Power on a national or global scale is often expressed in gigawatts, each one of which is equal to a billion watts or even terawatts, where a terawatt is equal to a trillion watts.
The science that deals with energy in its various forms and with work is thermodynamics. There are some important laws of thermodynamics. The first law of thermodynamics states that energy is neither created nor destroyed. This law is also known as the law of conservation of energy. As an example of the application of this law, consider Figure 15.1. The energy associated with moving earth enters the system as chemical energy in the form of diesel fuel, and the oxygen from the air required for its combustion. This is a valuable form of concentrated chemical energy that can be used to propel a tractor or locomotive, in a turbine attached to a generator for the generation of electrical energy, or as a fuel to generate heat in an oil-fired furnace. The fuel is burned in the tractor’s engine, and more than half of its energy is dissipated as heat to the surroundings. The rest is used to move the tractor and dirt. The energy originally contained in a concentrated useful form in the diesel fuel is not destroyed, but it is dissipated in a dilute form, mostly to warm the surroundings very slightly.
The first law of thermodynamics must always be kept in mind in the practice of green chemistry. The best practice of green chemistry and, indeed, of all environmental science, requires the most efficient use of energy as it goes through a system. The availability of energy is often the limiting factor in using and recycling materials efficiently.


