14.10: Direct Biosynthesis of Polymers
- Page ID
- 285725
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Cellulose in wood and cotton is only one example of the numerous significant polymers that are made biologically by organisms. Other important examples are wool and silk, which are protein polymers. A big advantage of these kinds of polymers from an environmental viewpoint is that polymers made biologically are also the ones that are most likely to be biodegradable. Attempts have been made to synthesize synthetic polymers that are biodegradable, These efforts have centered on those prepared from biodegradable monomers, such as lactic acid.
From the standpoint of green chemistry, it is ideal to have polymers that are made by organisms in a form that is essentially ready to use. Recently, interest has focussed on poly (hydroxyalkanoate) compounds, of which the most common are polymers of 3-hydroxybutyricacid:
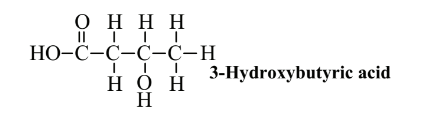
This compound and related ones have both a carboxylic acid (-CO2H) and an alcohol (-OH) group. As discussed in Section 6.4 and shown in Reaction 6.4.1, a carboxylic acid can bond with an alcohol with the elimination of a molecule of H2O forming an ester linkage. Since the hydroxyalkanoates have both functional groups, the molecules can bond with each other to form polymer chains:
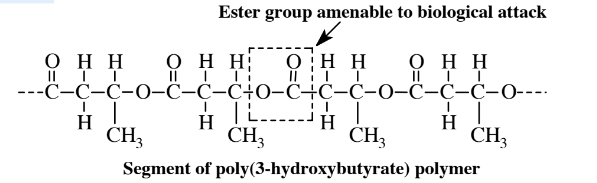
Ester groups are among the most common in a variety of biological compounds, such as fats and oils, and organisms possess enzyme systems that readily attack ester linkages. Therefore, the poly(hydroxyalkanoate) compounds are amenable to biological attack. Aside from their biodegradability, polymers of 3-hydroxybutyric acid and related organic acids that have -OH groups on their hydrocarbon chains (alkanoates) can be engineered to have a variety of properties ranging from rubberlike to hard solid materials.
It was first shown in 1923 that some kinds of bacteria make and store poly(hydroxy-alkanoate) ester polymers as a reserve of food and energy. In the early 1980s it was shown that these materials have thermoplastic properties, meaning that they melt when heated and resolidify when cooled. This kind of plastic can be very useful, and the thermoplastic property is rare in biological materials. One commercial operation was set up for the biological synthesis of a polymer in which 3-hydroxybutyrate groups alternate with 3-hydroxyvalerate groups, where valeric acid has a 5-carbon atom chain. This process uses a bacterium called Ralstonia eutropia fed glucose and the sodium salt of propionic acid (structural formula in Figure 14.6) to make the polymer in fermentation vats. Although the process works, costs are high because of problems common to most microbial fermentation synthesis processes: The bacteria have to be provided with a source of food, yields are relatively low, and it is difficult to isolate the product from the fermentation mixture.
Developments in genetic engineering have raised the possibility of producing poly(hydroxyalkanoate) polymers in plants. The plant Arabidopsis thaliana has accepted genes from bacterial Alcaligenes eutrophus that have resulted in plant leaves containing as much as 14% poly(hydroxybutyric acid) on a dry weight basis. Transgenic Arabidopsis thaliana and Brassica napus (canola) have shown production of the copolymer of 3-hydroxybutyrate and 3-hydroxyvalerate. If yields can be raised to acceptable levels, plant-synthesized poly(hydroxyalkanoate) materials would represent a tremendous advance in biosynthesis of polymers because of the ability of photosynthesis to provide the raw materials used to make the polymers.


