12.10: Biodegradation
- Page ID
- 285707
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Bacteria, fungi, and protozoa in the environment play an important role in biodegrading both natural materials and synthetic substances. These processes occur predominantly in water, in sediments in bodies of water, and in soil. Biodegradation is the process by which biomass from deceased organisms is broken down to simple inorganic constituents, thus completing the cycle in which biomass is produced from atmospheric carbon dioxide and from water by photosynthesis.
The biodegradation of substances in the environment by the action of enzymes in microorganisms can be divided metabolically into two categories. The first of these is the utilization by microorganisms of organic matter that can be metabolized for energy and as material to synthesize additional biomass. This is the route taken by microorganims degrading biomass from other organisms, and to a lesser extent in the biodegradation of some xenobiotic materials.The second way in which microorganisms metabolize environmental chemicals is through cometabolism in which the organism’s enzymes act upon the substances as a “side-line” of their normal metabolic processes. The substances that are cometabolized are called secondary substrates because they are not the main compounds for which the enzymatic processes are designed.
A commonly cited example of cometabolism occurs with the action of Phanerochaete chrysosporium on organochlorine compounds, including PCBs and dioxins. Commonly known as the white rot fungus, this organism has an enzyme system that normally breaks down lignin, the degradation-resistant “glue” that holds cellulose together in wood and woody plants. Under certain stressed conditions, however, the enzyme will act to cometabolize synthetic organochlorine compounds and was once widely promoted as a means of remediating hazardous waste sites contaminated with such compounds.
The degree of biodegradation varies over a wide range. In the simplest case, the change to the substrate molecule is relatively minor, such as addition, deletion, or modification of a functional group. Complete biodegradation to simple inorganic species—CO2 for carbon, NH4+ or NO3- for nitrogen, HPO42- for phosphorus, SO42- for sulfur—is the process of mineralization, which is crucial in completing elemental cycles in the environment.
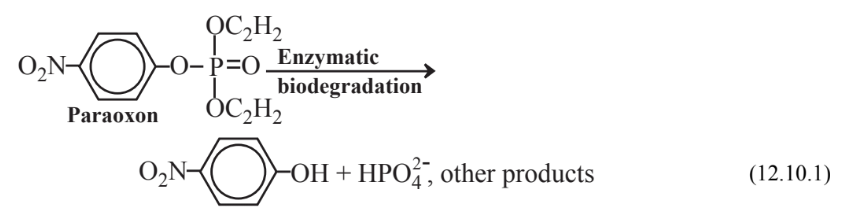
An important step in biodegradation is the modification of a substance to reduce its toxicity. This process is called detoxication (or often detoxification). An example of detoxication is given in Reaction 12.10.1 below for the conversion of insecticidal paraoxon, a potent nerve poison, to p-nitrophenol, which is only about 0.005× as toxic. In some cases, however, action of microorganisms in the environment may produce a much more toxic material. An example of this is the generation of highly toxic, mobile methylmercury species, Hg(CH3)2 and HgCH3+ from insoluble, relatively harmless inorganic mercury species.
A number of factors are involved in determining the effectiveness and rate of biodegradation.The compound in question has to be biodegradable. Biodegradability is influenced by both physical properties, such as water solubility, and chemical characteristics including the presence of functional groups amenable to microbial attack. As illustrated by the example, below,
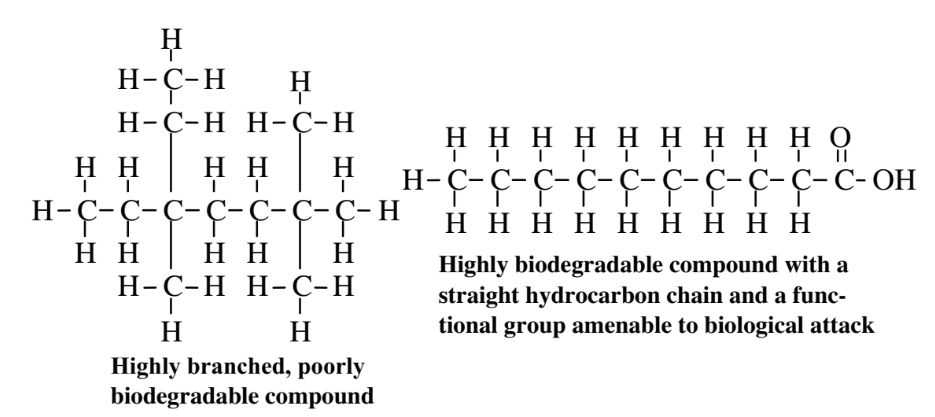
branched-chain hydrocarbons are very resistant to biodegradation whereas straight-chain hydrocarbons, especially those with a suitable functional group, are readily metabolized by microorganisms. It should be noted that even very poorly biodegradable compounds can often be degraded under suitable conditions. As an example, phenol,
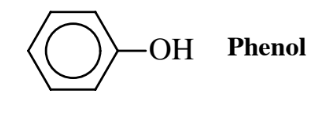
is a biocidal compound that kills bacteria and was once the most commonly used disinfectant. However, in dilute solution and under the appropriate conditions, phenol can be destroyed by bacteria. An important aspect of biodegradation of resistant compounds is to use microorganisms acclimated to the particular kind of compounds. Populations of acclimated microorganisms are commonly found in locations where the kinds of compounds to be treated have been spilled, such as in petroleum spills on soil.
Biodegradability of compounds is an important consideration in green chemistry. This is especially true of “consumable” materials that are dissipated to the environment. One of the most common examples of the use of a biodegradable material as a consumable material is the substitution in household detergents of biodegradable LAS surfactant, which has a readily biodegradable straight hydrocarbon chain as part of its molecular structure, in place of ABS surfactant, which has a poorly biodegradable branched chain.


