11.2: The Nature of the Geosphere
- Page ID
- 285356
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Defined in Section 8.4 and illustrated in Figure 8.5, the geosphere consists of the rocks, minerals, soils, sediments, molten magma, and pressurized gases and liquids beneath Earth’s surface. The Deepwater Horizon incident discussed above speaks to human efforts to extract from the geosphere the materials and energy that modern civilization demands and it illustrates the potential environmental hazards associated with exploiting the geosphere’s resources. It speaks to the need for understanding the geosphere and treating it with the respect that it demands for the maintenance of the environment under living conditions.
Most of our food is grown on the geosphere and humans extract from it metals, fossil fuels, fertilizers for plants, and a variety of minerals used in construction and for other purposes. Over the years, huge quantities of waste products have been discarded to the geosphere, in some cases very carelessly in a manner that poses threats to humans and other organisms. A thin layer on top of the geosphere — in places only a few centimeters deep — composes topsoil which supports the plant life upon which humans and most other land-dwelling organisms depend for their food.
The geosphere interacts strongly with the other environmental spheres. Streams and rivers flow through channels in the geosphere, lakes and reservoirs occupy cavities on the surface of the geosphere, and groundwater occurs in aquifers underground that are part of the geosphere. Gases are exchanged between the geosphere and the atmosphere, light and infrared radiation transmitted through the atmosphere warm the surface of ground, and it in turn radiates back to the atmosphere the infrared radiation by which Earth loses the energy it absorbs from the sun.
The geosphere is tied to green chemistry in many important respects, including the following
- Plants that provide most food for humans and animals grow on the geosphere.
- Plants growing on the geosphere already provide, and have the potential to provide much more, biomass for use as renewable materials, such as wood, fiber, raw materials, and fuel.
- The geosphere is the source of nonrenewable minerals, ores, fossil fuels, and other materials used by modern industrialized societies.
- Modifications and alterations of the geosphere have profound effects upon the environment.
- Sources of fresh water are stored in lakes and rivers on the surface of the geosphere, move by means of streams, rivers, and canals on the geosphere, and occur in aquifers underground.
- The geosphere is the ultimate sink for disposal of a variety of wastes.
This chapter briefly addresses the nature of the geosphere, and resource utilization from the geosphere. Waste disposal on land or underground are considered in later chapters. Because of the special importance of soil and the plants that grow on it as sources of food and fiber, soil is discussed in some detail.
Physical Nature of the Geosphere
At the center of Earth is an iron-rich inner core, hot enough to be molten under normal pressures, but compressed to a solid by the enormous pressures at such great depths. Surrounding this core is an outer core consisting of molten rock called magma. Earth’s solid outer layer consists of the mantle and the crust, a layer that is only 5-40 km thick. Only the upper layers of the crust are accessible to humans.
For the most part, the crust consists of rocks, which in turn are made up of minerals characterized by a definite chemical composition and crystal structure. Only about 25 of the approximately 2000 known minerals compose most rocks. Because most of the crust consists of chemically combined oxygen (49.5%) and silicon (25.7%), the most abundant minerals are silicates composed of various silicon oxides, examples of which are quartz, SiO2, and potassium feldspar, KAlSi3O8. Other elements in Earth’s crust are aluminum (7.4%, commonly occurring as Al2O3), iron (4.7% as Fe3O4 and other iron oxides), calcium (3.6% in limestone, CaCO3, and dolomite, CaCO3•MgCO3), sodium (2.8%), potassium (2.6%), and magnesium (2.1%). That leaves only 1.6% of the crust to serve as a source of other important mineral substances, including metals other than iron and aluminum, phosphorus required for plant growth, and sulfur widely used in industrial applications.
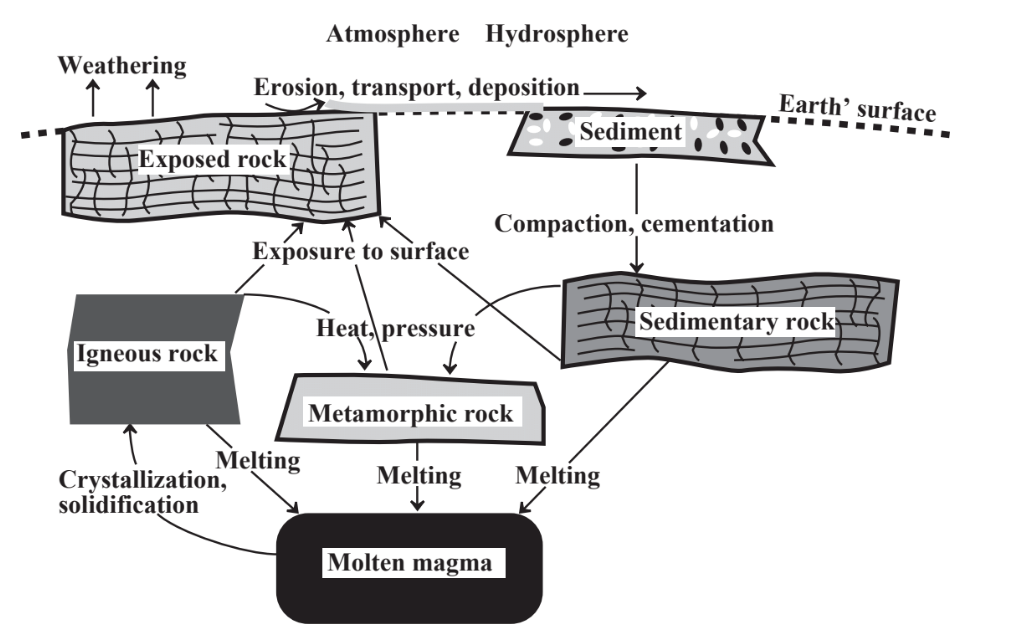
The rocks that compose Earth’s crust participate in the rock cycle shown in Figure 11.1. Igneous rock is rock that has solidified from molten rock called magma that has penetrated to near Earth’s surface. Exposed to water, atmospheric oxygen, and various organisms, igneous rock becomes highly altered, reaching a state of greater physical and chemical equilibrium with the atmosphere. This is a process called weathering. Weathering products end up as soil and are carried by water to be deposited as sediments. Sediments that become buried and compressed become secondary minerals, among the most abundant of which are clays, consisting of silicon and aluminum oxides, produced by the weathering of minerals such as potassium feldspar, KAlSi3O8. A common clay is kaolinite, Al2Si2O5(OH)4.
Although the earth’s crust is very thin compared to Earth’s total diameter, there is an even much thinner, fragile and vitally important layer covering the crust —soil. Soil is the finely divided mixture of mineral and organic matter upon which plants grow, providing the food that humans and most other animals eat. Productive soil may be only a few centimeters thick, and rarely is more than a few meters in thickness. Soil is uniquely important and of crucial importance in sustainability. Humans are capable of inflicting great damage on soil causing it to become unproductive and in extreme cases resulting in widespread hunger and even starvation.
Geochemistry is the branch of chemistry that deals with rocks and minerals and the chemical interactions of the geosphere with other environmental spheres. The specialized branch of geochemistry relating to environmental influences and interactions of the geosphere is environmental geochemistry. Weathering by chemical processes is a particularly important aspect of geochemistry. Almost imperceptible under dry conditions, weathering proceeds at a much more rapid rate in the presence of water. The rate of weathering is also increased by the action of microorganisms, some of which secrete chemical species that attack rock and leach nutrients from it. Particularly important to weathering are lichens, which are algae and fungi living together synergistically. The algae utilize solar energy to convert atmospheric carbon dioxide to plant biomass and the fungi utilize the biomass and anchor the organisms to the rock surface and extract nutrients from it.
Human Influences
Human activities have a tremendous influence on the geosphere as evidenced by hills leveled, valleys filled in, and vast areas paved to make freeways, parking lots, and shopping centers. One such influence is on surface albedo, defined as the percentage of impinging solar energy reflected back from Earth’s surface. The surface albedo of an asphalt paved surface is only about 8%. Amore alarming effect is desertification in which normally productive soil is converted to unproductive desert in areas where rainfall is marginal. This phenomenon is discussed in more detail in Section 11.10.


