10.11: Photochemical Smog
- Page ID
- 285695
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)One of the most common urban air pollution problems is the production of photochemical smog. This condition occurs in dry, stagnant air masses, usually stabilized by a temperature inversion (see Figure 8.1), that are subjected to intense sunlight. A smoggy atmosphere contains ozone, O3, organic oxidants, nitrogen oxides, aldehydes, and other noxious species. In latter stages of smog formation visibility in the atmosphere is lowered by the presence of a haze of fine particles formed by the oxidation of organic compounds in smog.
The chemical ingredients of smog are nitrogen oxides and organic compounds, both released from the automobile, as well as from other sources. The driving energy force behind smog formation is electromagnetic radiation with a wavelength at around 400 nm or less, in the ultraviolet region, just shorter than the lower limit for visible light. Energy absorbed by a molecule from this radiation can result in the formation of active species, thus initiating photochemical reactions.
Although methane, CH4, is one of the least active hydrocarbons in terms of forming smog, it will be used here to show the smog formation process because it is the simplest hydrocarbon molecule. Smog is produced in a series of chain reactions. The first of these occurs when a photon of electromagnetic radiation with a wavelength less than 398 nm is absorbed by a molecule of nitrogen dioxide.
\[\ce{NO2 } h \nu \rightarrow \ce{NO + O}\]
to produce an oxygen atom, O. The oxygen atom is a very reactive species that can abstract a hydrogen atom from methane,
\[\ce{CH4 + O \rightarrow HO \cdot + H3C \cdot}\]
to produce a methyl radical, H3C•, and a hydroxyl radical, HO•. In these formulas, the dot shows a single unpaired electron. A chemical species with such a single electron is a free radical. The hydroxyl radical is especially important in the formation of smog and in a wide variety of other kinds of photochemical reactions. The methyl radical can react with an oxygen molecule,
\[\ce{H3C \cdot + O2 \rightarrow H3COO \cdot}\]
to produce a methylperoxyl radical, H3COO•. This is a strongly oxidizing, reactive species. One of the very important reactions of peroxyl radicals is their reaction with NO, produced in the photochemical dissociation of NO2 (see Reaction 8.10.1 above),
\[\ce{NO + H3COO \cdot \rightarrow NO2 + H3COO \cdot}\]
To regenerate NO2, which can undergo photodissociation, re-initiating the series of chain reactions by which smog is formed. Literally hundreds of other reactions can occur, leading eventually to oxidized organic matter that produces the small particulate matter characteristic of smog.
As the process of smog formation occurs, numerous noxious intermediates are generated. One of the main ones of these is ozone, O3, and it is the single species most characteristic of smog. Whereas ozone is an essential species in the stratosphere, where it filters out undesirable ultraviolet radiation, it is a toxic species in the troposphere that is bad for both animals and plants. Another class of materials formed with smog consists of oxygen-rich organic compounds containing nitrogen of which peroxyacetyl nitrate, PAN,
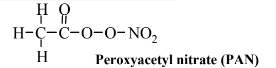
is the most common example. This compound and ones similar to it are potent oxidizers and highly irritating to eyes and mucous membranes of the respiratory tract. Also associated with smog are aldehydes, which are irritants to eyes and the respiratory tract. The simplest aldehyde, and one commonly found in smoggy atmospheres, is formaldehyde:

Harmful Effects of Smog
Smog adversely affects human health and comfort, plants, materials, and atmospheric quality. Each of these aspects is addressed briefly here. Ozone is the smog constituent that is generally regarded as being most harmful to humans, plants, and materials, although other oxidants and some of the noxious organic materials, such as aldehydes, are harmful as well. People exposed to 0.15 parts per million of ozone in air experience irritation to the respiratory mucous tissues accompanied by coughing, wheezing, and bronchial constriction. These effects may be especially pronounced for individuals undergoing vigorous exercise because of the large amounts of air that they inhale. On smoggy days, air pollution alerts may advise against exercise and outdoor activities. Because of these effects, the U.S. Environmental Protection Agency recommends an 8-hour standard limit for ozone of 0.075 ppm and is considering further lowering the standard. In a smoggy atmosphere, the adverse effects of ozone are aggravated by exposure to other oxidants and aldehydes.
Plants are harmed by exposure to nitrogen oxides, ozone, and peroxyacetyl nitrate (PAN, see above), all oxidants present in a smoggy atmosphere. PAN is the most harmful of these constituents, damaging younger plant leaves, especially. Ozone exposure causes formation of yellow spots on leaves, a condition called chlorotic stippling. Some plant species, including sword-leaf lettuce, black nightshade, quickweed, and double-fortune tomato, are extremely susceptible to damage by oxidant species in smog and are used as bioindicators of the presence of smog. Costs of crop and orchard damage by smog run into millions of dollars per year in areas prone to this kind of air pollution, such as southern California.
Materials that are adversely affected by smog are generally those that are attacked by oxidants. The best example of such a material is rubber, especially natural rubber, which is attacked by ozone. Indeed, the hardening and cracking of natural rubber has been used as a test for atmospheric ozone.
Visibility-reducing atmospheric aerosol particles are the most common manifestation of the harm done to atmospheric quality by smog. The smog-forming process occurs by the oxidation of organic materials in the atmosphere, and carbon-containing organic materials are the most common constituents of the aerosol particles in an atmosphere afflicted by smog. Conifer trees(pine and cypress) and citrus trees are major contributors to the organic hydrocarbons that are precursors to organic particle formation in smog.
Preventing Smog with Green Chemistry
Smog is basically a chemical problem, which would indicate that it should be amenable to chemical solutions. Indeed, the practice of green chemistry and the application of the principles of industrial ecology can help to reduce smog. This is due in large part to the fact that a basic premise of green chemistry is to avoid the generation and release of chemical species with the potential to harm the environment. The best way to prevent smog formation is to avoid the release of nitrogen oxides and organic vapors that enable smog to form. At an even more fundamental level, measures can be taken to avoid the use of technologies likely to release such substances, for example, by using alternatives to polluting automobiles for transportation.
The evolution of automotive pollution control devices to reduce smog provides an example of how green chemistry can be used to reduce pollution. The first measures taken to reduce hydrocarbon and nitrogen oxide emissions from automobiles were very much command-and-control and “end-of-pipe” measures. These primitive measures implemented in the early 1970s did reduce emissions, but with a steep penalty in fuel consumption and in driving performance of vehicles. However, over the last three decades, the internal combustion automobile engine has evolved into a highly sophisticated computer-controlled machine that generally performs well, emits few air pollutants, and is highly efficient. (And it would be much more efficient if those drivers who feel that they must drive “sport utility” behemoths would switch to vehicles of a more sensible size.) This change has required an integrated approach involving reformulation of gasoline. The first major change was elimination from gasoline of tetraethyllead, an organometallic compound that poisoned automotive exhaust catalysts (and certainly was not good for people). Gasoline was also reformulated to eliminate excessively volatile hydrocarbons and unsaturated hydrocarbons (those with double bonds between carbon atoms) that are especially reactive in forming photochemical smog.
An even more drastic approach to eliminating smog-forming emissions is the use of electric automobiles that do not burn gasoline. These vehicles certainly do not pollute as they are being driven, but they suffer from the probably unsolvable problem of a very limited range between charges and the need for relatively heavy batteries. However, hybrid automobiles using a small gasoline or diesel engine that provides electricity to drive electric motors propelling the automobile and to recharge relatively smaller batteries can largely remedy the emission and fuel economy problems with automobiles. The internal combustion engine on these vehicles runs only as it is needed to provide power and, in so doing, can run at a relatively uniform speed that provides maximum economy with minimum emissions.
Another approach that is being used on vehicles as large as buses that have convenient and frequent access to refueling stations is the use of fuel cells that can generate electricity directly from the catalytic combination of elemental hydrogen and oxygen, producing only harmless water as a product (see Chapter 16). There are also catalytic process that can generate hydrogen from liquid fuels, such as methanol, so that vehicles carrying such a fuel can be powered by electricity generated in fuel cells.
Green chemistry can be applied to devices and processes other than automobiles to reduce smog-forming emissions. This is especially true in the area of organic solvents used for parts cleaning and other industrial operations, vapors of which are often released to the atmosphere. The substitution of water with proper additives or even the use of supercritical carbon dioxide fluid can eliminate such emissions.


