10.9: Acid Rain
- Page ID
- 285693
Along with hydrogen chloride, HCl, emitted to the atmosphere by the combustion of chlorine-containing organic compounds, sulfur dioxide and nitrogen oxides react in the atmosphere to produce strongly acidic H2SO4 and HNO3, respectively. Incorporated into rainwater, these acids fall to the ground as acid rain. A more general term, acid deposition, refers to the effects of atmospheric strong acids, acidic gases (SO2), and acidic salts (NH4NO3 and NH4HSO4). Acid deposition is a major air pollution problem.
Figure 10.7 shows a typical distribution of acidic precipitation in the 48 contiguous U.S. states. This figure illustrates that acidic precipitation is a regional air pollution problem, not widespread enough to be a global problem, but spreading beyond local areas. (There have been some unfortunate cases where localized release of acid, usually as sulfur dioxide from metal ore smelting operations have affected local areas, often devastating vegetation within several kilometers of the source
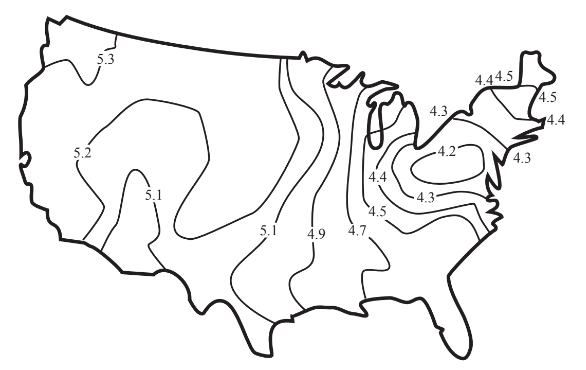
Transport processes that move atmospheric acids and their precursor acid gases from their sources to downwind areas are very important in determining areas affected by acid rain. The northeastern U.S. and southeastern Canada are affected by acid originating from stack gas emissions carried by prevailing southwesterly winds from Missouri, Illinois, Kentucky and other regions to the southwest. Southern Norway, Sweden, and Finland receive acid precipitation originating farther south in Europe.
Numerous adverse effects have been reported as the result of acidic precipitation. These can be divided into the following major categories:
• Direct effects upon the atmosphere manifested by reduced and distorted visibility. These effects are due to the presence of sulfuric acid droplets and solutions or solid particles of acidic salts, such as NH4HSO4.
• Phytotoxicity (toxicity to plants) and destruction of sensitive forests. These effects can be direct, resulting from exposure of plant leaves and roots to acidic precipitation and to acid-forming gases, particularly SO2 and NO2. They can also be indirect, primarily by the liberation of phytotoxic Al3+ ion by the action of acidic rainfall on soil.
• Direct effects on humans and other animals. These are usually respiratory effects, and asthmatics are especially vulnerable.
• Effects upon plants and fish (especially fish fingerlings) in acidified lake water where the lake is not in contact with minerals, particularly CaCO3, capable of neutralizing acid.
• Damage to materials. Stone (especially acid-soluble limestone and marble) and metal used in building can be corroded and etched by acidic precipitation. Electrical equipment, particularly relay contacts and springs can be corroded by acidic precipitation.
• Some measures can be taken to mitigate the effects of acid rain, although these are very limited once the pollutant has formed. Some success has been achieved with treating acidified lakes with pulverized limestone to neutralize acid. Corrosion-resistant materials can be used in applications where exposure to acid rain is likely. Protective coatings, such as corrosion-resisting paint primers on metals, can be applied to materials likely to be exposed to acidic precipitation. But the best protection is to prevent formation and release of SO2 and NOx gases leading to acid rain formation by measures described in the preceding section.


