10.6: The Enormous Importance of Climate
- Page ID
- 285352
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
The most important aspect of the atmosphere for humans and other living beings is climate. Climate consists of long-term trends in weather and varies a lot over Earth’s surface. For example, the climate in desert regions of the world may be relatively hot and dry, but the weather in such regions may at times produce torrential rainfall or frigid temperatures. Of all the things that humans might do to irreversibly and catastrophically harm Mother Earth, the only home that they have or ever can have, the potential to change climate is the most serious. It is well established that climate has changed markedly in past times, most notably during several ice ages that each lasted for thousands of years. Volcanic eruptions noted in historical accounts have caused temporary cooling of the atmosphere and widespread hunger when summer growing seasons were cut short as a result. Tree-ring data indicate centuries-long droughts in parts of the world in the past, which resulted in the decline of some civilizations in the present-day U.S. Southwest, for example. Although these were natural events, recent weather data indicate changes in microclimate due to human activities. For example, some urban areas in Southeast Asia have become darker in recent decades due to particulate air pollution.
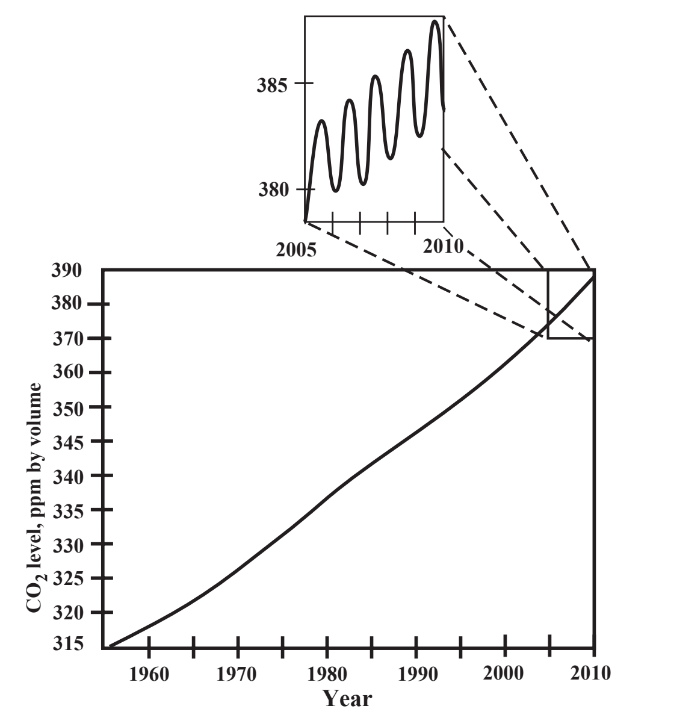
Of all the things that humans may be doing that could change climate, emissions of large quantities of gases that cause atmospheric warming are the most serious. Of these, carbon dioxide, CO2, is the most important. Carbon dioxide is a normal constituent of the atmosphere, essential as a source of carbon for plant photosynthesis. Along with water vapor and other trace gases, atmospheric carbon dioxide absorbs outgoing infrared radiation from Earth, thus keeping the planet’s surface temperature at a tolerable level. Levels of carbon dioxide gas in the atmosphere are now about 390 parts per million by volume. This represents an almost 50% increase overestimated pre-industrial concentrations of 260 ppm. Furthermore, as shown by the plot in Figure 10.4, global CO2 levels are increasing by about 1 ppm per year, and ice core evidence indicates that these levels were only about 200 ppm at the peak of the last ice age around 18,000 years ago. So, humans are clearly increasing atmospheric carbon dioxide levels significantly, largely through the combustion of carbon-containing fossil fuels and as the result of destruction of forests. The importance of photosynthesis in determining atmospheric carbon dioxide is illustrated by the inset in Figure 10.4 showing an annual fluctuation of about 5 ppm CO2 in the northern hemisphere attributed to photosynthesis. The minimum in this cycle occurs around September at the end of the summer growing season and the maximum occurs around April as photosynthesis is getting underway after winter.
The concern with increasing carbon dioxide in the atmosphere is that it will lead to — indeed is leading to — an excess of a good thing, warming of the global atmosphere. This is the now well known greenhouse effect, which may not become as dire as some experts predict, but which has a real possibility of becoming the worst environmental problem so far created by humans.
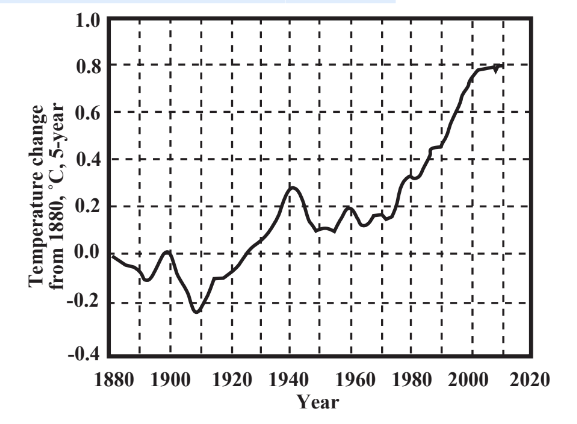
Is Earth warming? Sophisticated computer models indicate that it is backed by evidence from increasingly accurate temperature records over more than 100 years. These temperature records have been especially accurate over the last several decades because they have been read over all Earth’s surface by satellite. Global temperatures analyzed by the Goddard Institute for Space Studies (GISS) in New York City show that the 1980s were the warmest decade on record globally since reasonably accurate global temperatures have been measured. The 1980s were followed by a warmer decade in the 1990s and the warmest decade of all from the beginning of 2000 to the end of 2009 (see Figure 10.5). The hottest year ever documented historically was 2005 with 1998, 2002, 2003, 2006, 2007, and 2009 all essentially tied for second.
Gases other than carbon dioxide may be involved in greenhouse warming. These include chlorofluorocarbons and N2O. The one most likely to cause a problem is methane, CH4, which has increased from estimated pre-industrial atmospheric levels of 0.70 ppm, to present values of l.8ppm. Although these values are much lower than those of carbon dioxide, each methane molecule is 20–30 times more effective in trapping heat than is each CO2 molecule. A number of humana ctivities have contributed to the release of methane. Part of this is due to leakage of natural gas, which consists of methane, and from release as a byproduct of petroleum production. The 2010 blowout of the Deepwater Horizon well in the Gulf of Mexico released large quantities of methane that had been sequestered largely in combination with water as solid methane hydrates far below the ocean floor. These methane-containing structures belong to a class of materials called clathrates, an inherently unstable network of host molecules containing open cavities. In the case of methane hydrates the clathrates are clusters of approximately 46 H2O molecules that enclose molecules of CH4. Methane hydrates can form and be stable under pressure at temperatures significantly above the freezing point of water. Estimates are that more methane is contained in these structures globally than in all the deposits of natural gas. A major concern with global warming is that methane contained in methane hydrates held by permafrost will be released causing a feedback loop that will put even more methane into the atmosphere and result in even greater warming. Bacteria growing in the absence of oxygen in municipal refuse landfills, in rice paddies, and in the stomachs of ruminant animals (cows, sheep, moose) release enormous quantities of methane.
Although there could be some benefits of mild global warming, the net effect would almost certainly be bad, perhaps catastrophic. Climate models predict an average global temperature increase of 1.5–5 ̊C. That does not sound like much, but it is about as much again as the temperature increase that occurred from the last ice age until now. Especially if the warming is toward the high side of the projected range, it would greatly affect climate and rainfall. The melting of the polar and Greenland ice caps along with expansion of warmer ocean water would cause sea levels to rise as much as 0.5–1.5 meters. Decreased rainfall and increased water evaporation would contribute to severe drought and water shortages that could make some currently popular areas of the world virtually uninhabitable.
Can Green Chemistry Help Deal With Global Warming?
Green chemistry and the related area of industrial ecology can help deal with the problem of global warming in two major respects. The first approach is to provide means to prevent global warming from taking place. The second approach is in coping with global warming, if it occurs.
The prevention of global warming is best accomplished by avoiding the release of potential greenhouse gases. As noted above, the most significant of these is carbon dioxide. One way to reduce the release of carbon dioxide is by using biomass as fuel or raw material for the manufacture of various products. Burning a biomass fuel does release carbon dioxide to the atmosphere, but an exactly equal amount of carbon dioxide was removed from the atmosphere in the photosynthetic process by which the biomass was made, so there is no net addition of CO2. Unless or until biomass-derived materials used in feedstocks are burned or biodegraded, their use represents a net loss of carbon dioxide from the atmosphere.
Another potential use of green chemistry to prevent addition of carbon dioxide to the atmosphere is through carbon sequestration in which carbon dioxide is produced, but is bound in a form such that it is not released to the atmosphere. This approach has the greatest potential in applications where the carbon dioxide is produced in a concentrated form. In Chapter 16, reactions are shown by which carbon from coal is reacted with oxygen and water to produce elemental hydrogen and carbon dioxide. The net reaction for this production is the following:
\[\ce{2C + O2 + 2H2O \rightarrow 2CO2 + 2H2}\]
The hydrogen generated can be used as a pollution-free fuel in fuel cells or combustion engines. The carbon dioxide can be pumped into deep ocean waters, although this has the potential to lower ocean pH slightly, which would be detrimental to marine organisms. Another option is to pump the carbon dioxide deep underground. A side benefit of the latter approach is that in some areas carbon dioxide pumped underground can be used to recover additional crude oil from depleted oil-bearing formations.
An indirect green chemistry approach to the reduction of carbon dioxide emissions is to develop alternative methods of energy production. One thing that would be very beneficial is the development of more efficient photovoltaic cells. These devices have become marginally competitive for the generation of electricity, and even relatively small improvements in efficiency would enable their much wider use, replacing fossil fuel sources of electricity generation. Another device that would be extremely useful is a system for the direct photochemical dissociation of water to produce elemental hydrogen and oxygen, which could be used in fuel cells. An application of green biochemistry that would reduce carbon dioxide emissions is the development of plants with much higher efficiencies for photosynthesis. Plants now are only about 0.5%efficient in converting light energy to chemical energy. Raising this value to only 1% would make a vast difference in the economics of producing biomass as a substitute for fossil carbon.
Green chemistry can also be applied in the prevention of release of greenhouse gases other than carbon dioxide, especially ultrastable volatile compounds that have a high greenhouse gas potential. An excellent example of green chemistry has been the replacement of chlorofluorocarbons (Freons) with analogous compounds having at least one C-H bond, that are rather readily destroyed in the troposphere. Although this was done to prevent destruction of stratospheric ozone by chlorofluorocarbons, it has been useful to reduce greenhouse warming. Both kinds of compounds act as greenhouse gases, but those with at least one C-H bond last for much shorter times during which they are available to absorb infrared radiation. As discussed inSection 10.10, green chemistry can be applied to avoid generating extremely stable sulfurhexafluoride, SF6, and completely fluorinated hydrocarbons, such as CF4.
Another approach is to limit the emissions of methane, CH4. Large quantities of methane are released by anoxic bacteria growing in flooded rice paddies. By developing strains of rice and means of cultivation that enable the crop to be grown on unflooded soil, this source of methane can be greatly reduced. Methane collection systems placed in municipal waste landfills can prevent the release of methane from this source and provide a source of methane fuel.
Green chemistry, biochemistry, and biology can be used to deal with global warming when it occurs. Crops, fertilizers, and pesticides can be developed that enable plants to grow under the drought conditions that would follow global warming. Another approach is the development of salt-tolerant crops that can be grown on soil irrigated with saline water, where fresh water supplies are limited.


