8.3: The Atmosphere
- Page ID
- 285327
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
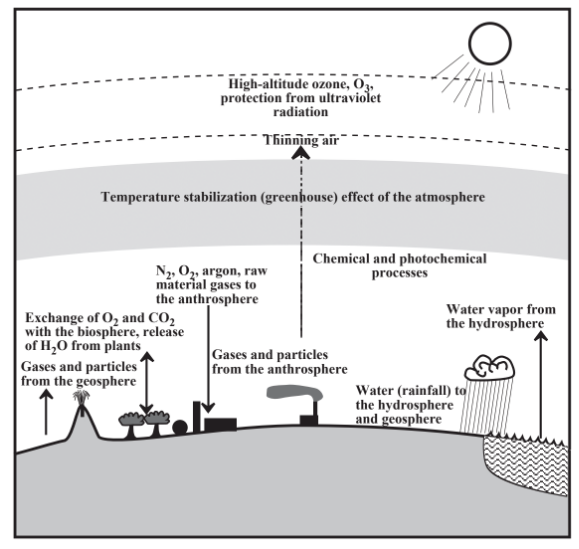
Illustrated in Figure 8.4, the atmosphere having a total mass of about 5.15×1015 tons is a layer of gases blanketing Earth that diminishes rapidly in density with increasing altitude. More than 99% of the atmosphere’s mass is within 40 kilometers (km) of Earth’s surface, with the majority of the air below 10 km altitude (compared to Earth’s diameter of almost 13,000 km). A person exposed to air at the approximately 13,000 m altitude at which commercial jet aircraft fly could remain conscious for only about 15 seconds without supplementary oxygen. There is no clearly defined upper limit to the atmosphere, which keeps getting thinner with increasing altitude. A practical upper limit may be considered to be an altitude of about 1000 km above which airmolecules can be lost to space (a region called the exosphere).
The atmosphere nurtures life on Earth in many important respects. Some of the main ones of these are the following:
• The atmosphere constitutes much of Earth’s natural capital because of its attributes listed below.
• The atmosphere is a source of molecular O2 for all organisms that require it including humans and all other animals. In addition, pure oxygen, argon, and neon are extracted from the atmosphere for industrial uses.
• At approximately 0.039% carbon dioxide, CO2, the atmosphere is the source of carbon that plants and other photosynthetic organisms use to synthesize biomass
• Consisting mostly of molecular N2, the atmosphere serves as a source of nitrogen that is an essential component of protein and other biochemicals as well as a constituent of a variety of synthetic chemicals. Organisms “fix” this nitrogen in the biosphere chemically by the action of bacteria such as Rhizobium and it is fixed synthetically in the anthrosphere under much more severe conditions of temperature and pressure.
•The atmosphere acts as a blanket to keep Earth’s surface at an average temperature of about 15 ̊C at sea level and within a temperature range that enables life to exist(the “good” greenhouse effect).
•Earth’s atmosphere absorbs very short wavelength ultraviolet radiation from the sun and space, which, if it reached organisms on Earth’s surface, would tear apart the complex biomolecules essential for life. In this respect, the stratospheric ozone layer is of particular importance
• The atmosphere contains and carries water vapor evaporated from oceans that forms rain and other kinds of precipitation over land in the hydrologic cycle(Figure 8.1)
The atmosphere is 1–3% by volume water vapor, a level that is somewhat higher in tropical regions and lower in desert areas and at higher altitudes where condensation to liquid droplets and ice crystals removes water vapor. Exclusive of water vapor, on a dry basis air is 78.1% by volume nitrogen gas (N2), 21.0% O2, 0.9% noble gas argon, and almost 0.039% CO2, a level that keeps increasing by a little more than 0.001% per year. In addition, there are numerous trace gases in the atmosphere at levels below 0.002% including ammonia, carbon monoxide, helium, hydrogen, krypton, methane, neon, nitrogen dioxide, nitrous oxide, ozone, sulfur dioxide, and xenon.
Figure 8.4 shows some of the main features and aspects of the atmosphere and its relationship to other environmental spheres. Except for a few aviators who fly briefly into the stratosphere, living organisms experience the lowest layer called the troposphere characterized by decreasing temperature and density with increasing altitude. The troposphere extends from surface level, where the average temperature is 15 ̊C, to about 11 km (the approximate cruising altitude of commercial jet aircraft) where the average temperature is –56 ̊C (the tropopause discussed in Section 8.2)
Above the troposphere is the stratosphere in which the average temperature increases from about –56 ̊C at its lower boundary to about –2 ̊C at its upper limit. The stratosphere is warmed by energy of intense solar radiation impinging on air molecules. Because this radiation can break the bonds holding O2 molecules together, at higher altitudes the stratosphere maintains a significant level of O atoms and of ozone (O3) molecules formed by combination of O atoms with O2 molecules. Stratospheric ozone molecules are essential for the ability of humans and other organisms to exist on Earth’s surface because of their ability to filter out damaging ultraviolet radiation before it can penetrate to Earth’s surface. Although the stratosphere’s ozone is spread over many km in altitude, it is commonly called the ozone layer. If all this ozone that is so essential for life were in a single layer of pure ozone at conditions near Earth’s surface, it would be only about 3 millimeters thick! Some classes of chemical species, especially the chlorofluorocarbons or Freons formerly used as refrigerants, are known to react in ways that destroy stratospheric ozone, and their elimination from commerce has been one of the major objectives of efforts in achieving sustainability.
Above the stratosphere are the atmospheric mesosphere and thermosphere. which are relatively less important in the discussion of the atmosphere. Radiation energetic enough to tear electrons away from atmospheric molecules and atoms reaches these regions giving rise to a region containing ions called the ionosphere.
Earth’s atmosphere is crucial in absorbing, distributing, and radiating the enormous amount of energy that comes from the sun. A square meter of surface directly exposed to sunlight unfiltered by air would receive energy from the sun at a power level of 1,340 watts. Called the solar flux, this level of power impinging on just one square meter could power an electric iron or thirteen 100-watt light bulbs plus a 40-watt bulb! If one considers Earth’s cross-sectional area, the rate of total incoming solar energy is huge. Incoming solar radiation is in the form of electromagnetic radiation, which has a wavelike character in which shorter wavelengths are more energetic. The incoming radiation in the form electromagnetic radiation centered in the visible wavelength region with a maximum intensity at a wavelength of 500 nanometers (1 nm = 10-9m) is largely absorbed and converted to heat in the atmosphere and at Earth’s surface.
On average, the incoming solar energy must be balanced with heat energy radiated back into space; otherwise Earth would have melted and vaporized long ago. The outbound heat energy radiates into space as infrared radiation between about 2 micrometers and 40 μm (1 μm = 10-6 m)with a maximum intensity at about 10 μm). This energy is delayed on its outward path by being re-absorbed by water molecules, carbon dioxide, methane, and other minor species in the atmosphere. This has a warming (greenhouse) effect that is very important in sustaining life on Earth. As discussed in Chapter 10, anthrospheric discharges of greenhouse gases, especially carbon dioxide and methane, are likely causing an excessive greenhouse effect, which will have harmful effects on global climate.
Climate
Largely determined by conditions in the atmosphere, climate is crucial to the well-being of humans and other organisms on Earth. Weather refers to such factors as rain, wind, cloud cover, atmospheric pressure, and temperature whereas climate involves these conditions over a long period of time such as the warm, low-humidity weather that prevails in southern California or the cool, generally rainy conditions of Ireland. Meteorology is the science of the atmosphere and weather and climate is addressed by climatology.
Much of the driving force behind weather and climate is due to the fact that the incoming flux of solar energy is very intense in regions around the equator and very low in polar regions due to the angles at which the solar flux impinges Earth in these regions. Heated equatorial air tends to expand and flow away from the equatorial regions, creating winds and carrying with it large quantities of energy and water vapor evaporated from the oceans. As the air cools, water vapor condenses forming precipitation and warming the air from heat released when water goes from a vapor to a liquid. This process is the driving force behind hurricanes and typhoons which can result in torrential rainfalls and damaging winds.
Meteorological phenomena have an important influence on air pollution. An important example occurs with temperature inversions in which a layer of relatively warm air confines a surface layer of somewhat cooler, more dense stagnant air close to the ground. Hydrocarbons and nitrogen oxides confined in the stagnant air mass are acted upon by solar energy to cook up the noxious mixture of ozone, oxidants, aldehydes and other unpleasant materials that constitute photochemical smog.
Winds are important in air pollution. The lack of substantial wind is required for the formation of photochemical smog. Sulfur dioxide emitted to the atmosphere may be relatively innocuous near the point of discharge but transformed to harmful acid rain as it is carried some distance by wind. That is the reason that New England and parts of Canada can be afflicted with acid rain from sulfur dioxide given off by coal-fired power plants some distance away in the Ohio River Valley.
Climate and sustainability go hand-in-hand. Favorable climate conditions and a relatively unpolluted atmosphere are important parts of natural capital. A favorable climate is required to maintain food productivity. One of the greatest concerns with the emission of excessive amounts of greenhouse gases to the atmosphere is warming that will result in catastrophic drought in formerly productive agricultural regions leading to much reduced food production and even widespread starvation.
Anthrospheric Influences on the Atmosphere
Human activities in the anthrosphere strongly influence the atmosphere and have enormous potential to affect Earth’s environment as a whole. Photochemical smog with its destructive ozone and other oxidants along with other pollutants and visibility-obscuring atmospheric particulate matter results when nitrogen oxides and reactive hydrocarbons, largely from internal combustion vehicle engine exhausts, are emitted to the atmosphere from the anthrosphere. The sulfur dioxide and nitrogen oxide precursors to strong sulfuric and nitric acids in acid rain come from anthrospheric activities associated with fossil fuel combustion and other sources such as roasting of sulfide metal ores. Because of factors such as these, one of the most important aspects of sustainability is the construction and operation of the anthrosphere in ways that preserve the quality of the atmosphere. One of the important aspects of green chemistry is to use products and processes that do not contribute to damage to the atmosphere.


