7.8: Biochemical Processes in Metabolism
- Page ID
- 285323
So far, this chapter has discussed the cells in which biochemical processes occur, the major categories of biochemicals, and the enzymes that catalyze biochemical reactions. Biochemical processes involve the alteration of biomolecules, their synthesis, and their breakdown to provide the raw materials for new biomolecules, processes that fall under the category of metabolism. Metabolic processes may be divided into the two major categories of anabolism (synthesis) and catabolism (degradation of substances). An organism may use metabolic processes to yield energy or to modify the constituents of biomolecules. Metabolism is discussed here as it affects biochemicals and in Chapter 12, Section 12.4, as it applies to the function of organisms in the biosphere.
Metabolism is a very important consideration in green chemistry and sustainability. Toxic substances that impair metabolism pose a danger to humans and other organisms and attempts are made to avoid such substances in the practice of green chemistry. Exposures to environmental pollutants that impair metabolism endanger humans and other organisms; the control of such pollutants is an important aspect of environmental chemistry. Metabolic processes are used to make renewable raw materials and to modify substances to give desired materials. The complex metabolic process of photosynthesis provides the food that forms the base of essentially all food webs and is increasingly being called upon to provide renewable raw materials for manufacturing.
Energy-Yielding Processes
The processing of energy is obviously one of the most important metabolic functions of organisms. The metabolic processes by which organisms acquire and utilize energy are complex generally involving numerous steps and various enzymes. Organisms can process and utilize energy by one of three major processes:
- Respiration in which organic compounds undergo catabolism
- Fermentation, which differs from respiration in not having an electron transport chain
- Photosynthesis, in which light energy captured by plant and algal chloroplasts is used to synthesize sugars from carbon dioxide and water
There are two major pathways in respiration. Oxic respiration(called aerobic respiration in the older literature) requires molecular oxygen whereas anoxic respiration (anaerobic respiration) occurs in the absence of molecular oxygen. Oxic respiration uses the Krebs cycle to obtain energy from the following reaction:
\[\ce{C6H12O6 + 6O2 \rightarrow 6CO2 + 6H2O + energy}\]
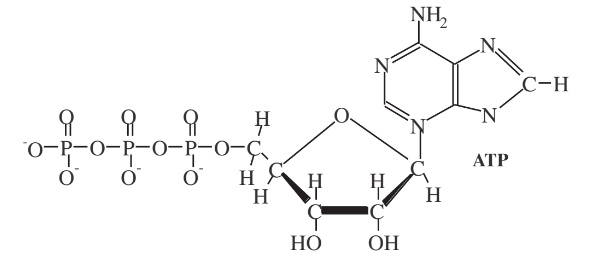
About half of the energy released is converted to short-term stored chemical energy, particularly through the synthesis of adenosine triphosphate(ATP) shown in Figure 7.10
The highly energized ATP molecule is sometimes described as the “molecular unit of currency” for the transfer of energy within cells during metabolism. It releases its energy when it loses a phosphate group and reverts to adenosine diphosphate and other precursors. ATP is used by enzymes and proteins for cell processes including biosynthetic reactions (anabolism), cell division, and motility (such as occurs in moving protozoa cells). In so doing, ATP is continually being produced and reconverted back to its precursor species in an organism. Some studies have suggested that the human body processes its own mass in ATP during a single day! Whereas ATP is used for very short term energy storage and processing, for longer-term energy storage,glycogen or starch polysaccharides are synthesized, and for still longer-term energy storage, lipids(fats) are generated and retained by the organism.
As noted above, fermentation differs from respiration in not having an electron transport chain and organic compounds are the final electron acceptors rather than O2in the energy-yielding process. Many biochemical processes including some used to make commercial products are fermentations. A common example of fermentation is the production of ethanol from sugars by yeasts growing in the absence of molecular oxygen:
\[\ce{C6H12O6 \rightarrow 2CO2 + 2C2H5OH}\]
Photosynthesis, is an energy-capture process in which light energy captured by plant and algal chloroplasts is used to synthesize sugars from carbon dioxide and water:
\[\ce{6CO2 + 6H2O + h \nu \rightarrow C6H12O6 + 6O2}\]
When it is dark, plants cannot get the energy that they need from sunlight but still must carry on basic metabolic processes using stored food. Plant cells, like animal cells, contain mitochondria in which stored food is converted to energy by cellular respiration.
Nonphotosynthetic organisms depend upon organic matter produced by plants for their food and are said to be heterotrophic. They act as “middlemen” in the chemical reaction between oxygen and food material using the energy from the reaction to carry out their life processes Plant cells, which use sunlight as a source of energy and CO2 as a source of carbon, are classified as autotrophic. In contrast, animal cells must depend upon organic material manufactured by plants for their food. These are called heterotrophic cells.
Biochemical conversions involving energy are very important in the practice of green chemistry and sustainability. The most obvious connection is the capture of solar energy as chemical energy by photosynthesis. As discussed in Chapter 15, photosynthetically produced biomass can serve as a source of chemically fixed carbon for the synthesis of chemical fuels including synthetic natural gas, gasoline, diesel fuel, and ethanol. A tantalizing possibility is to use recombinant DNA techniques to increase by several-fold the very low efficiency of photosynthesis by most plants. Fermentation has a strong role to play in sustainable energy development. As shown in Reaction 7.8.2, fermentation of glucose produces ethanol, which can be used as fuel.
Anoxic fermentation of biomass (abbreviated {CH2O}) from sources such as sewage sludge or food wastes yields methane (natural gas) the cleanest-burning of all hydrocarbon fuels:
\[\ce{2(CH2O) \rightarrow CH4 + CO2}\]


