7.3: Carbohydrates
- Page ID
- 285318
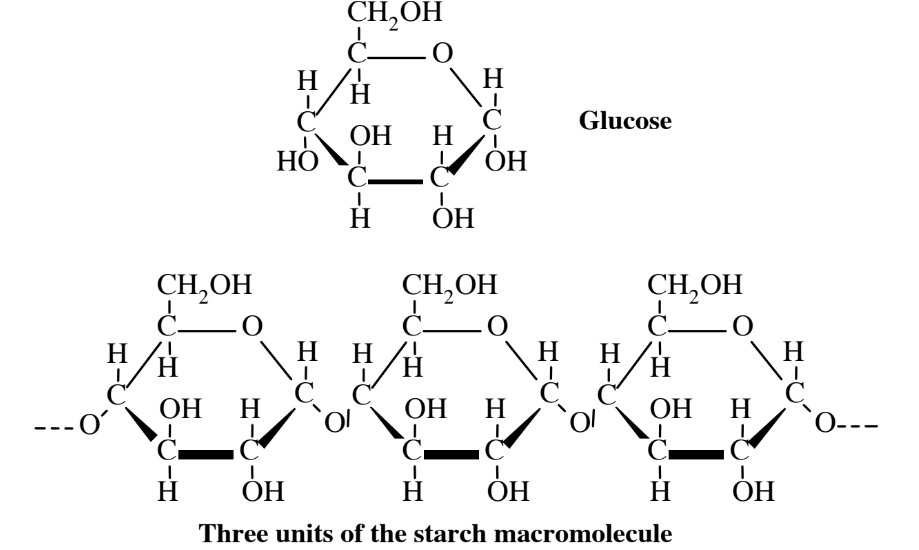
Carbohydrates are biomolecules consisting of carbon, hydrogen, and oxygen having the approximate simple formula CH2O. One of the most common carbohydrates is the simple sugar glucose shown in Figure 7.2. Units of glucose and other simple sugars called monosaccharides join together in chains with the loss of a water molecule for each linkage to produce macromolecular polysaccharides. These include starch and cellulose in plants and starch-like glycogen in animals.
Glucose carbohydrate is the biological material generated from water and carbon dioxide when solar energy in sunlight is utilized in photosynthesis. The overall reaction is
\[\ce{6CO2 + 6H2O \rightarrow C6H12O6 + 6O2}\]
This is obviously an extremely important reaction because it is the one by which inorganic molecules are used to synthesize high-energy carbohydrate molecules that are in turn converted to the vast number of biomolecules that comprise living systems. There are other simple sugars, including fructose, mannose, and galactose, that have the same simple formula as glucose, C6Η12Ο6, but which must be converted to glucose before being utilized by organisms for energy. Common table sugar, sucrose, C12Η22Ο11, consists of a molecule of glucose and one of fructose linked together (with the loss of a water molecule); because it is composed of two simple sugars sucrose is called a disaccharide.
Starch molecules, which may consist of several hundred glucose units joined together, are readily broken down by organisms to produce simple sugars used for energy and to produce biomass. For example, humans readily digest starch in potatoes or bread to produce glucose used for energy (or to make fat tissue).
The chemical formula of starch is (C6H10O5)n, where n may represent a number as high as several hundreds. What this means is that the very large starch molecule consists of as many as several hundred units of C6H10O5 from glucose joined together. For example, if n is 100, there are 6 times 100 carbon atoms, 10 times 100 hydrogen atoms, and 5 times 100 oxygen atoms in the molecule. Its chemical formula is C600H1000O500. The atoms in a starch molecule are actually present as linked rings represented by the structure shown in Figure 7.2. Starch occurs in many foods, such as bread, potatoes, and cereals. It is readily digested by animals, including humans.
Cellulose is a polysaccharide which is also made up of C6H10O5 units. Molecules of cellulose are huge, with molecular masses of around 400,000. The cellulose structure (Figure 7.3) is similar to that of starch. Cellulose is produced by plants and forms the structural material of plant cell walls. Wood is about 60% cellulose, and cotton contains over 90% of this material. Fibers of cellulose are extracted from wood and pressed together to make paper.
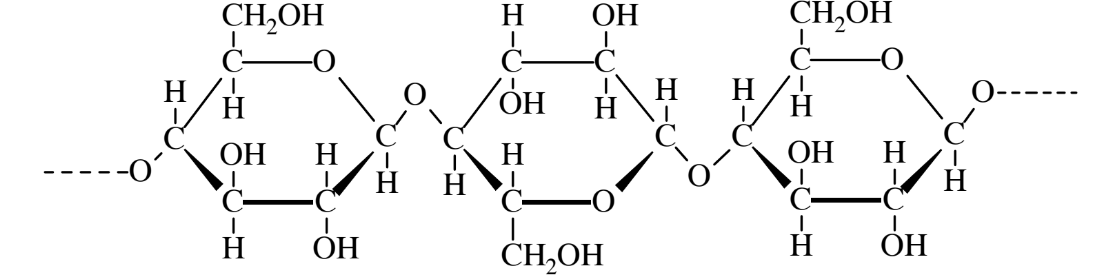
Humans and most other animals cannot digest cellulose because they lack the enzyme needed to hydrolyze the oxygen linkages between the glucose molecules. Ruminant animals (cattle, sheep, goats, moose) have bacteria in their stomachs that break down cellulose into products which can be used by the animal. Fungi and termites existing synergistically with cellulose-degrading bacteria biodegrade huge quantities of cellulose. Chemical processes are available to convert cellulose to simple sugars by the reaction
\[\underbrace{\ce{(C6H10O5)_{n}}}_{\textbf{cellulose}} \ce{ + nH2O} \rightarrow \underbrace{\ce{nC6H12O6}}_{\textbf{glucose}}\]
where n may be 2000-3000. This involves breaking the linkages between units of C6H10O5 by adding a molecule of H2O at each linkage, a hydrolysis reaction. Large amounts of cellulose from wood, sugar cane, and agricultural products go to waste each year. The hydrolysis of cellulose enables these products to be converted to sugars, which can be fed to animals. The potential for producing very large quantities of glucose from cellulose have led to intense efforts to hydrolyze cellulose to glucose with enzymes (biological catalysts) and is an important effort in green chemistry.
Carbohydrates are potentially very important in green chemistry. For one thing, they are a concentrated form of organic energy synthesized and stored by plants as part of the process by which plants capture solar energy through photosynthesis. Carbohydrates can be utilized directly for energy or fermented to produce ethanol, C2Η6O, a combustible alcohol that is added to gasoline or can even be used in place of gasoline. Secondly, carbohydrates are a source of organic raw material that can be converted to other organic molecules to make plastics and other useful materials.


