2.14: The Nature of Matter and States of Matter
- Page ID
- 284423
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We are familiar with matter in different forms. We live in an atmosphere of gas that is mostly N2 with about 1/4 as much oxygen, O2, by volume. We only become aware of the gas in the atmosphere when something is wrong with it, such as contamination by irritating air pollutants. A person stepping into an atmosphere of pure N2 would not notice anything wrong immediately, but would die within a few minutes, not because N2 is toxic, but because the atmosphere lacks life-giving oxygen. The same atmosphere that we breathe contains water in the gas form as water vapor. And we are also familiar, of course, with liquid water and with solid ice.
he air that we breathe, like most substances, is a mixture consisting of two or more substances. Air is a homogeneous mixture meaning that the molecules of air are mixed together at a molecular level. There is no way that we can take air apart by simple mechanical means, such as looking at it under a magnifying glass and picking out its individual constituents. Another common substance that is a homogeneous mixture is drinking water, which is mostly H2O molecules, but which also contains dissolved O2 and N2 from air, dissolved calcium ions (Ca2+), chlorine added for disinfection, and other materials.
A heterogeneous mixture is one that contains discernible and distinct particles that, in principle at least, can be taken apart mechanically. Concrete is a heterogeneous mixture. Careful examination of a piece of broken concrete shows that it contains particles of sand and rock embedded in solidified Portland cement.
A material that consists of only one kind of substance is known as a pure substance. Absolutely pure substances are almost impossible to attain. Hyperpure water involved in semiconductor manufacturing operations approaches absolute purity. Another example is 99.9995% pure helium gas used in a combination gas chromatograph/mass spectrometer instrument employed for the chemical analysis of air and water pollutants.
Mixtures are very important in the practice of green chemistry. Among other reasons why this is so is that separation of impurities from mixtures in the processing of raw materials and in recycling materials is often one of the most troublesome and expensive aspects of materials utilization and may generate large quantities of wastes. Impurities may make mixtures toxic. For example, toxic arsenic, which is directly below phosphorus in the periodic table and has chemical properties similar to phosphorus, occurs as an impurity in the phosphate ores from which elemental phosphorus is extracted. This is not a problem for phosphorus used as fertilizer because the small amount of arsenic added to the soil is negligible compared to the arsenic naturally present in the soil. But, if the phosphorus is to be made into phosphoric acid and phosphate salts to be added to soft drinks or to food, impurity arsenic cannot be tolerated because of its toxicity requiring removal of this element at considerable expense.
Many byproducts of manufacturing operations are mixtures. For example, organochlorine solvents used to clean and degrease machined parts are mixtures that contain grease and other impurities. As part of the process for recycling these solvents, the impurities must be removed by expensive processes such as distillation. The separation of mixture constituents is often one of the most expensive aspects of the recycling of materials.
States of Matter
As shown in Figure \(\PageIndex{1}\), the three common states of matter are gases, liquids, and solids. These are readily illustrated by water, the most familiar form of which is liquid water. Ice is a solid and water vapor in the atmosphere or in a steam line is a gas.
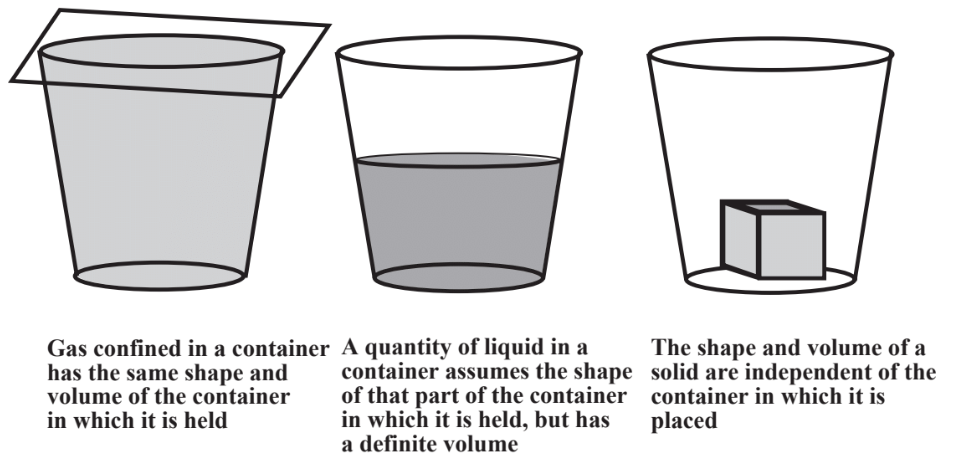
Gases, such as those composing the air around us, are composed mostly of empty space through which molecules of the matter composing the gas move constantly, bouncing off each other or the container walls millions of times per second. A quantity of gas expands to fill the container in which it is placed. Because they are mostly empty space, gases can be significantly compressed; squeeze a gas and it responds with a decreased volume. Gas temperature is basically an expression of the tendency of the gas molecules to move more rapidly; higher temperatures mean faster molecular movement and more molecules bouncing off each other or container walls per second. The constant impact of gas molecules on container walls is the cause of gas pressure. Because of the free movement of molecules relative to each other and the presence of mostly empty space, a quantity of gas takes on the volume and shape of the container in which it is placed. The physical behavior of gases is described by several gas laws relating volumes of gas to quantities of the gas, pressure, and temperature. Calculations involving these laws are covered at the beginning of Chapter 10.
Molecules of liquids can move relative to each other, but cannot be squeezed together to a significant extent, so liquids are not compressible. Liquids do take on the shape of the part of a container that they occupy. Molecules of solids occupy fixed positions relative to each other. Therefore, solids cannot be significantly compressed and a solid object retains its shape regardless of the container in which it is placed.


