2.12: Combining Atoms to Make Molecules and Compounds
- Page ID
- 284421
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)About the only atoms that exist alone are those of the noble gases, a group of elements including helium, neon, argon, and radon located on the far right of the periodic table. Even the simple hydrogen atom in the elemental state is joined together with another hydrogen atom. Two or more uncharged atoms bonded together are called a molecule. As illustrated in Figure \(\PageIndex{1}\), the hydrogen molecule consists of 2 hydrogen atoms as denoted by the chemical formula of elemental hydrogen, H2. This formula states that a molecule of elemental hydrogen consists of 2 atoms of hydrogen, shown by the subscript of 2. The atoms are joined together by a chemical bond. As explained in Chapter 3, a hydrogen atom consists of a very small positively charged nucleus surrounded by a much larger cloud of negative charge from a single, rapidly moving, electron. But, hydrogen atoms are more “content” with 2 electrons. So two hydrogen atoms share their two electrons constituting the chemical bond in the hydrogen molecule. A bond composed of shared electrons is a covalent bond.
Chemical Compounds
The example just discussed was one in which atoms of the same element, hydrogen, join together to form a molecule. Most molecules consist of atoms of different elements joined together. An example of such a molecule is that of water, chemical formula H2O. This formula stands for the fact that the water molecule consists of two hydrogen atoms bonded to one oxygen atom, O, where the absence of a subscript number after the O indicates that there is 1 oxygen atom. The water molecule is shown in Figure \(\PageIndex{2}\). Each of the hydrogen atoms is held to the oxygen atom in the water molecule by two shared electrons in a covalent bond. A material such as water in which two or more elements are bonded together is called a chemical compound. It is because of the enormous number of combinations of two or more atoms of different elements that it is possible to make 20 million or more chemical compounds from fewer than 100 elements.

Ionic Bonds
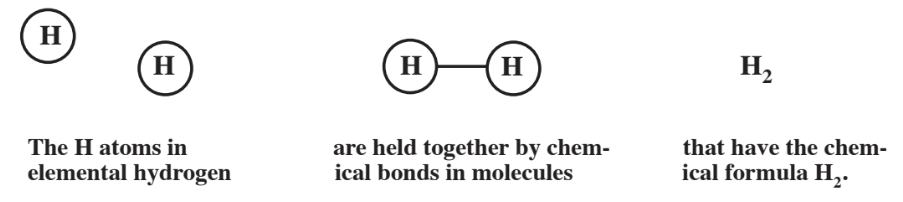
Two different molecules have just been discussed in which atoms are joined together by covalent bonds consisting of shared electrons. Another way in which atoms can be joined together is by transfer of electrons from one atom to another. A single neutral atom has a number of electrons surrounding its nucleus that is the same as the number of protons in the nucleus of the atom. But, if the atom loses one or more negatively charged electrons, it ends up with a net positive electrical charge and the atom becomes a positively charged cation. An atom that has gained one or more negatively charged electrons attains a net negative charge and is called an anion. Cations and anions are attracted together in an ionic compound because of their opposite electrical charges. The oppositely charged ions are joined by ionic bonds in a crystalline lattice.
Figure \(\PageIndex{3}\) shows the best known ionic compound, sodium chloride, NaCl (common table salt). The chemical formula of NaCl implies that there is 1 Na for each Cl. In this case these consist of Na+ cations and Cl- anions. For ionic compounds such as NaCl, the first part of the name is simply that of the metal forming the cation, in this case sodium. The second part of the name is based upon the anion, but has the ending ide. So the ionic compound formed from sodium and chlorine is sodium chloride. As shown by the preceding example, ionic compounds may consist of ions composed of atoms that have lost electrons (producing positively charged cations) and other atoms that have gained electrons (producing negatively charged anions).
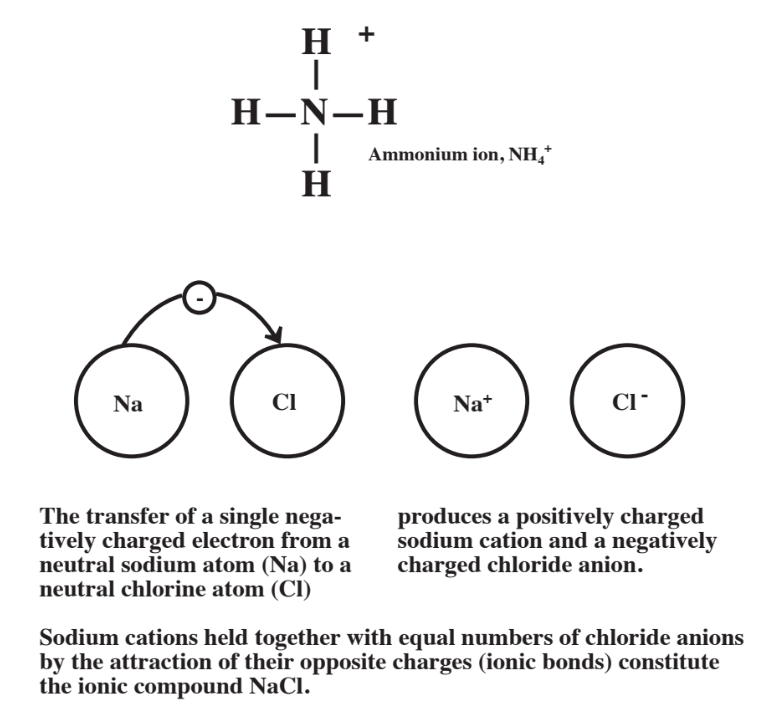
In addition to being charged atoms, ions may also consist of groups of several atoms with a net charge. Ammonium ion, NH4+, is such an ion. As shown below, the NH4+ cation consists of 4H atoms covalently bonded (by 2 shared electrons) to a central N atom, with the group of 5 total atoms having a net electrical charge of +1.