2.7: Reduction of Risk- Hazard and Exposure
- Page ID
- 284416
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A major goal in the manufacture and use of commercial products, and, indeed, in practically all areas of human endeavor, is the reduction of risk. There are two major aspects of risk — the hazard presented by a product or process and exposure of humans or other potential targets to those hazards.
\[\textrm{Risk = F{hazard x exposure}}\]
This relationship simply states that risk is a function of hazard times exposure. It shows that risk can be reduced by a reduction of hazard, a reduction of exposure, and various combinations of both.
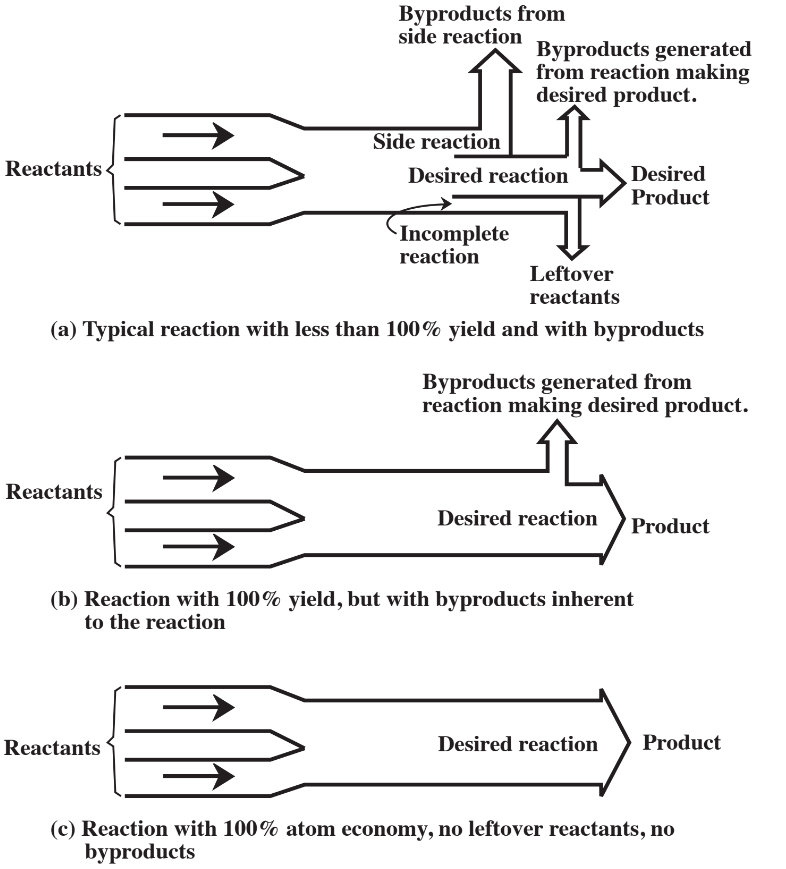
The command and control approach to reducing risk has concentrated upon reduction of exposure. Such efforts have used various kinds of controls and protective measures to limit exposure. The most common example of such a measure in the academic chemistry laboratory is the wearing of goggles to protect the eyes. Goggles will not by themselves prevent acid from splashing into the face of a student, but they do prevent the acid from contacting fragile eye tissue. Explosion shields will not prevent explosions, but they do retain glass fragments that might harm the chemist or others in the vicinity.
Reduction of exposure is unquestionably effective in preventing injury and harm. However, it does require constant vigilance and even nagging of personnel, as any laboratory instructor charged with making laboratory students wear their safety goggles at all times will attest. It does not protect the unprotected, such as a visitor who may walk bare-faced into a chemical laboratory ignoring the warnings for required eye protection. On a larger scale, protective measures may be very effective for workers in a chemical manufacturing operation but useless to those outside the area or the environment beyond the plant walls who do not have protection. Protective measures are most effective against acute effects, but less so against long-term chronic exposures that may cause toxic responses over many years period of time. Finally, protective equipment can fail and there is always the possibility that humans will not use it properly.
Where feasible, hazard reduction is a much more certain way of reducing risk than is exposure reduction. The human factors that play so prominently in successfully limiting exposure and that require a conscious, constant effort are much less crucial when hazards have been reduced. Compare, for example, the use of a volatile, flammable, somewhat toxic organic solvent used for cleaning and degreasing of machined metal parts with that of a water solution of a nontoxic cleaning agent used for the same purpose. To safely work around the solvent requires an unceasing effort and constant vigilance to avoid such hazards as formation of explosive mixtures with air, presence of ignition sources that could result in a fire, and excessive exposure by inhalation or absorption through skin that might cause peripheral neuropathy (a nerve disorder) in workers. Failure of protective measures can result in a bad accident or serious harm to worker health. The water-based cleaning solution, however, would not present any of these hazards so that failure of protective measures would not create a problem.
Normally, measures taken to reduce risk by reducing exposure have an economic cost that cannot be reclaimed in lower production costs or enhanced value of product. Of course, failure to reduce exposure can have direct, high economic costs in areas such as higher claims for worker compensation. In contrast, hazard reduction often has the potential to substantially reduce operating costs. Safer feedstocks are often less costly as raw materials. The elimination of costly control measures can lower costs overall. Again, to use the comparison of an organic solvent compared to a water-based cleaning solution, the organic solvent is almost certain to cost more than the aqueous solution containing relatively low concentrations of detergents and other additives. Whereas the organic solvent will at least require purification for recycle and perhaps even expensive disposal as a hazardous waste, the water solution may be purified by relatively simple processes, and perhaps even biological treatment, then safely discharged as wastewater to a municipal wastewater treatment facility. It should be kept in mind, however, that not all low-hazard materials are cheap, and may be significantly more expensive than their more hazardous alternatives. And, in some cases, nonhazardous alternatives simply do not exist.


