Hydrogenation of Unsaturated Fats and Trans Fat
- Page ID
- 440
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In the late 1970’s the lipid hypothesis came in to existences stating that eating saturated fats leads to elevated LDL (Low Density Lipoprotein) which was perceived to be "bad cholesterol." This will result in coronary heart disease which is hardening and narrowing of arteries resulting in heart attack. Fats were eventually classified in to 2 categories: “healthy fats” and “unhealthy fats”. Unhealthy fats where perceived to be saturated fats and healthy fats where perceived to be unsaturated fats.
A meta-analysis of 72 studies with over 103,052 people have found no validity in the lipid hypothesis. The conclusion of the Meta-Analysis was,“In contrast to current recommendations, this systematic review found no evidence that saturated fat increases the risk of coronary disease, or that polyunsaturated fats have a cardio protective effect.”[1] Dietary fats play a critical role in human health. They help keep cells healthy, help with brain development, help with the use of fat soluble vitamins, and they help cushion organs protecting them against blunt trauma. Fats come in multiple forms, saturated, unsaturated and trans fats just to name a few.
Saturated fats are solid at room temperature due to their molecular shape. The term saturated is in reference to an sp3 carbon chain that has its remaining sp3 orbitals bonded with hydrogen atoms. Thus the term “saturated”. It’s “saturated” with hydrogen. Saturated fats have a chain like structure which allows them to stack very well forming a solid at room temperature. Unsaturated fats are not linear due to double bonded carbons which results in a different molecular shape because the sp2 carbons are trigonal planar, not tetrahedral (sp3 carbons) as the carbons are in saturated fats. This change in structure will cause the fat molecules to not stack very well resulting in fats that are liquid at room temperature. Butter is mostly saturated fat, that’s why it’s solid at room temperature. Olive Oil is liquid at room temperature, thus it’s an unsaturated fat. An unsaturated fat can be made in to a saturated fat via hydrogenation reactions.
Hydrogenation Reaction
Unsaturated fatty acids may be converted to saturated fatty acids by the relatively simple hydrogenation reaction. Recall that the addition of hydrogen to an alkene (unsaturated) results in an alkane (saturated). A simple hydrogenation reaction is:
\[\ce{H_2C=CH_2 + H_2 \rightarrow CH_3CH_3}\]
alkene plus hydrogen yields an alkane
Vegetable oils are commonly referred to as "polyunsaturated". This simply means that there are several double bonds present. Vegetable oils may be converted from liquids to solids by the hydrogenation reaction. Margarines and shortenings are "hardened" in this way to make them solid or semi-solids.
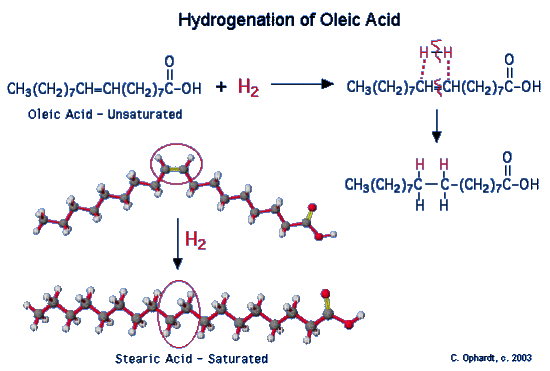
Figure 1: Hydrogenation of a oleic fatty acid
Vegetable oils which have been partially hydrogenated, are now partially saturated so the melting point increases to the point where a solid is present at room temperature. The degree of hydrogenation of unsaturated oils controls the final consistency of the product. What has happened to the healthfulness of the product which has been converted from unsaturated to saturated fats?
Trans Fat
A major health concern during the hydrogenation process is the production of trans fats. Trans fats are the result of a side reaction with the catalyst of the hydrogenation process. This is the result of an unsaturated fat which is normally found as a cis isomer converts to a trans isomer of the unsaturated fat. Isomers are molecules that have the same molecular formula but are bonded together differently. Focusing on the sp2 double bonded carbons, a cis isomer has the hydrogens on the same side. Due to the added energy from the hydrogenation process, the activation energy is reached to convert the cis isomers of the unsaturated fat to a trans isomer of the unsaturated fat. The effect is putting one of the hydrogens on the opposite side of one of the carbons. This results in a trans configuration of the double bonded carbons. The human body does not recognize trans fats.
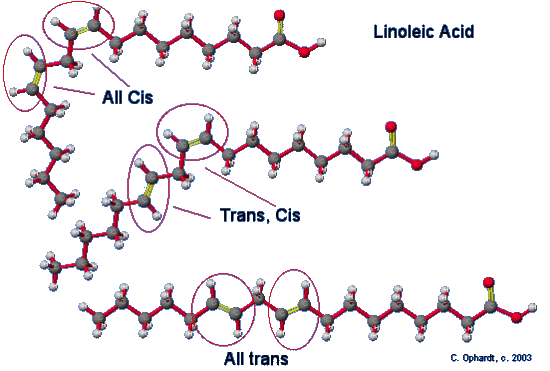
Although trans fatty acids are chemically "monounsaturated" or "polyunsaturated," they are considered so different from the cis monounsaturated or polyunsaturated fatty acids that they can not be legally designated as unsaturated for purposes of labeling. Most of the trans fatty acids (although chemically still unsaturated) produced by the partial hydrogenation process are now classified in the same category as saturated fats.
The major negative is that trans fat tends to raise "bad" LDL- cholesterol and lower "good" HDL-cholesterol, although not as much as saturated fat. Trans fat are found in margarine, baked goods such as doughnuts and Danish pastry, deep-fried foods like fried chicken and French-fried potatoes, snack chips, imitation cheese, and confectionery fats.
Contributors
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook,
- Antonio Rodriguez

