1.3: Fitting In
- Page ID
- 211465
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)death not-deaths deaths multiple deaths
dna dna (Deoxyribonucleic acid)
dying
Antibiotic Bacterium Death Gene
Deathstar
While it may seem obvious now, scientists did not always know that drugs have specific molecular targets in the body. In the mid-1880s, the French physiologist Claude Bernard made a crucial discovery that steered researchers toward understanding this principle. By figuring out how a chemical called curare works, Bernard pointed to the nervous system as a new focus for pharmacology. Curare—a plant extract that paralyzes muscles—had been used for centuries by Native Americans in South America to poison the tips of arrows. Bernard discovered that curare causes paralysis by blocking chemical signals between nerve and muscle cells. His findings demonstrated that chemicals can carry messages between nerve cells and other types of cells.
Since Bernard's experiments with curare, researchers have discovered many nervous system messengers, now called neurotransmitters. These chemical messengers are called agonists, a generic term pharmacologists use to indicate that a molecule triggers some sort of response when encountering a cell (such as muscle contraction or hormone release).
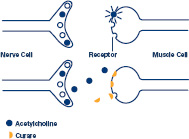
Nerve cells use a chemical messenger called acetylcholine (balls) to tell muscle cells to contract. Curare (half circles) paralyzes muscles by blocking acetylcholine from attaching to its muscle cell receptors.
The Right Dose
One of the most important principles of pharmacology, and of much of research in general, is a concept called "dose-response." Just as the term implies, this notion refers to the relationship between some effect—let's say, lowering of blood pressure—and the amount of a drug. Scientists care a lot about dose-response data because these mathematical relationships signify that a medicine is working according to a specific interaction between different molecules in the body.
Sometimes, it takes years to figure out exactly which molecules are working together, but when testing a potential medicine, researchers must first show that three things are true in an experiment. First, if the drug isn't there, you don't get any effect. In our example, that means no change in blood pressure. Second, adding more of the drug (up to a certain point) causes an incremental change in effect (lower blood pressure with more drug). Third, taking the drug away (or masking its action with molecule that blocks the drug) means there is no effect. Scientists most often plot data from dose-response experiments on a graph. A typical "dose-response curve" demonstrates the effects of what happens (the vertical Y-axis) when more and more drug is added to the experiment (the horizontal X-axis).

Dose-response curves determine how much of a drug ((X-axis) causes a particular effect, or a side effect, in the body (Y-axis).
One of the first neurotransmitters identified was acetylcholine, which causes muscle contraction. Curare works by tricking a cell into thinking it is acetylcholine. By fitting—not quite as well, but nevertheless fitting—into receiving molecules called receptors on a muscle cell, curare prevents acetylcholine from attaching and delivering its message. No acetylcholine means no contraction, and muscles become paralyzed.
Most medicines exert their effects by making physical contact with receptors on the surface of a cell. Think of an agonist-receptor interaction like a key fitting into a lock. Inserting a key into a door lock permits the doorknob to be turned and allows the door to be opened. Agonists open cellular locks (receptors), and this is the first step in a communication between the outside of the cell and the inside, which contains all the mini machines that make the cell run. Scientists have identified thousands of receptors. Because receptors have a critical role in controlling the activity of cells, they are common targets for researchers designing new medicines.
Curare is one example of a molecule called an antagonist. Drugs that act as antagonists compete with natural agonists for receptors but act only as decoys, freezing up the receptor and preventing agonists' use of it. Researchers often want to block cell responses, such as a rise in blood pressure or an increase in heart rate. For that reason, many drugs are antagonists, designed to blunt overactive cellular responses.
The key to agonists fitting snugly into their receptors is shape. Researchers who study how drugs and other chemicals exert their effects in particular organs—the heart, the lungs, the kidneys, and so on—are very interested in the shapes of molecules. Some drugs have very broad effects because they fit into receptors on many different kinds of cells. Some side effects, such as dry mouth or a drop in blood pressure, can result from a drug encountering receptors in places other than the target site. One of a pharmacologist's major goals is to reduce these side effects by developing drugs that attach only to receptors on the target cells.
That is much easier said than done. While agonists may fit nearly perfectly into a receptor's shape, other molecules may also brush up to receptors and sometimes set them off. These types of unintended, nonspecific interactions can cause side effects. They can also affect how much drug is available in the body.
Steroids for Surgery
In today's culture, the word "steroid" conjures up notions of drugs taken by athletes to boost strength and physical performance. But steroid is actually just a chemical name for any substance that has a characteristic chemical structure consisting of multiple rings of connected atoms. Some examples of steroids include vitamin D, cholesterol, estrogen, and cortisone—molecules that are critical for keeping the body running smoothly. Various steroids have important roles in the body's reproductive system and the structure and function of membranes. Researchers have also discovered that steroids can be active in the brain, where they affect the nervous system. Some steroids may thus find use as anesthetics, medicines that sedate people before surgery by temporarily slowing down brain function.
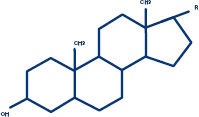
A steroid is a molecule with a particular chemical structure consisting of multiple "rings" (hexagons and pentagon, below).
Douglas Covey of Washington University in St. Louis, Missouri, has uncovered new roles for several of these neurosteroids, which alter electrical activity in the brain. Covey's research shows that neurosteroids can either activate or tone down receptors that communicate the message of a neurotransmitter called gammaaminobutyrate, or GABA. The main job of this neurotransmitter is to dampen electrical activity throughout the brain. Covey and other scientists have found that steroids that activate the receptors for GABA decrease brain activity even more, making these steroids good candidates for anesthetic medicines. Covey is also investigating the potential of neuroprotective steroids in preventing the nerve-wasting effects of certain neurodegenerative disorders.

