19.3: Why Are Micelles Uniform in Size?
- Page ID
- 294379
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Micelles are formed by amphiphiles that want to bury hydrophobic chains and expose charged head groups to water. Since a cavity must be formed for the micelle, the resulting surface tension of the cavity (the hydrophobic effect) results in the system trying to minimize its surface area, and thereby the number of molecules in the micelle. At the same time, the electrostatic repulsion between headgroups results in driving force to increase the surface area per headgroup. These competing effects result in an optimal micelle size.
We start by defining the chemical potential Δμn, which is the free energy per mole of amphiphilic molecule A to assemble a micelle with n molecules. Instead of using n, we will try to express the size of the micelle in terms of its surface area a and assume that it is spherical. Then, the free energy for forming a cavity for the micelle grows as γa, where γ is the surface tension. The surface area is expressed as an average surface area spanned by the charged headgroup of a monomer unit:
\[ a_e = a/n \]
 The repulsion term is hard to predict and depends on many variables. There are the electrostatic repulsions between head groups, but there is also the entropic penalty for forming the micelle that depends on size. As an approximation, we anticipate that the free energy should be inversely proportional to surface area. Then the free energy for forming a micelle with n molecules is
The repulsion term is hard to predict and depends on many variables. There are the electrostatic repulsions between head groups, but there is also the entropic penalty for forming the micelle that depends on size. As an approximation, we anticipate that the free energy should be inversely proportional to surface area. Then the free energy for forming a micelle with n molecules is
\[ \begin{aligned} \Delta G_n &= \gamma a + \dfrac{x}{a} \\ &= \gamma n a_e + \dfrac{x}{na_e} \end{aligned} \]
where x is a constant.
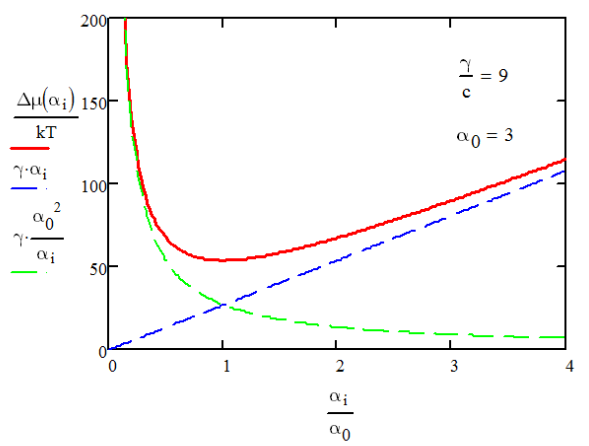
Solving for Δμ=∂ΔG/∂n, differentiating it with respect to ae, and setting to zero, we find the optimal micelle size, a0, is
\[ a_0 = \sqrt{\dfrac{x}{\gamma n^2}} \]
Solving for x and substituting in eq. (4), we obtain the chemical potential as:
\[ \Delta \mu = \dfrac{\gamma}{a_e}(a^2_e + a^2_0 ) = 2\gamma a_0 +\dfrac{\gamma}{a_e}(a_e-a_0)^2 \]
It has a parabolic shape with a minimum at a0.
Next, we can obtain the probability distribution for the micelle size as a function of head group surface area and aggregation number
\[ P_n = \exp (-n\Delta \mu /k_BT) \]
\[ P_n(a_e) ~\exp \left( - \dfrac{n\gamma (a_e - a_0)^2}{a_ek_BT} \right) \]
The relative populations of micelles are distributed in a Gaussian distribution about a0. The distribution of sizes has a standard deviation (or polydispersity) given by
\[ \sigma = \sqrt{\dfrac{na_ek_BT}{2\gamma}} \]
From a = 4πr2 = nae, we predict that the breadth of the micelle size distribution will scale linearly in the micelle radius, and as the square root of temperature and molecule number.
___________________________________________
K. Dill and S. Bromberg, Molecular Driving Forces: Statistical Thermodynamics in Biology, Chemistry, Physics, and Nanoscience. (Taylor & Francis Group, New York, 2010); J. N. Israelachvili, Intermolecular and Surface Forces, 3rd ed. (Academic Press, Burlington, MA, 2011), Ch. 20.


