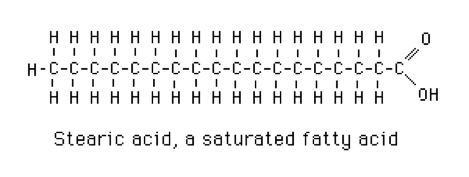3.1: Understanding Fats and Oils
- Page ID
- 93120
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Fats and oils are organic compounds that, like carbohydrates, are composed of the elements carbon (C), hydrogen (H), and oxygen (O), arranged to form molecules. There are many types of fats and oils and a number of terms and concepts associated with them, which are detailed further here.
Lipids
In baking, lipids are generally a synonym for fats. Baking books may talk about the “lipid content of eggs,” for example.
Triglycerides
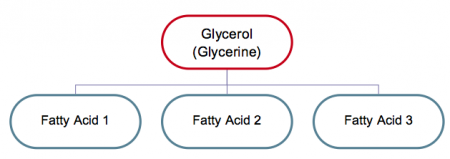
Triglycerides is another chemical name for the most common type of fats found in the body, indicating that they are usually made up of three (tri) fatty acids and one molecule of glycerol (glycerine is another name) as shown in Figure 3. (The mono and diglycerides that are used as emulsifiers have one and two fatty acids respectively.)
Figure 1 Composition of fats (triglycerides)
Fatty Acids
Each kind of fat or oil has a different combination of fatty acids. The nature of the fatty acid will determine the consistency of the fat or oil. For example, stearic acid is the major fatty acid in beef fat, and linoleic acid is dominant in seed oils. Fatty acids are defined as short, medium, or long chain, depending on the number of atoms in the molecule. The reason that some fat melts gradually is that as the temperature rises, each fatty acid will, in turn, soften, as its melting point is reached. Fats that melt all of a sudden mean that the fatty acids are of the same or similar type and have melting points within a narrow range. An example of such a fat is coconut fat: one second it is solid, the next, liquid.
Table 1 shows the characteristics of three fatty acids.
Table 1: Characteristics of Fatty Acids Type of Fatty Acid Melting Point Physical State (at room temperature) Stearic 69°C (157°F) Solid Oleic 16°C (61°F) Liquid Linoleic -12°C (9°F) Liquid
Rancid
Rancid is a term used to indicate that fat has spoiled. The fat takes on an unpleasant flavor when exposed to air and heat. Unsalted butter, for example, will go rancid quickly if left outside the refrigerator, especially in warm climates.
Oxidation/Antioxidants
Oxidation (exposure to air) causes rancidity in fats over time. This is made worse by combination with certain metals, such as copper. This is why doughnuts are never fried in copper pans!
Some oils contain natural antioxidants, such as tocopherols (vitamin E is one kind), but these are often destroyed during the processing. As a result, manufacturers add synthetic antioxidants to retard rancidity. BHA and BHT are synthetic antioxidants commonly used by fat manufacturers.
Saturated/Unsaturated
Saturated and unsaturated refer to the extent to which the carbon atoms in the molecule of fatty acid are linked or bonded (saturated) to hydrogen atoms. One system of fatty acid classification is based on the number of double bonds.
- 0 double bonds: saturated fatty acids. Stearic acid is a typical long-chain saturated fatty acid (Figure 2).[1]
Figure 2 Stearic Acid
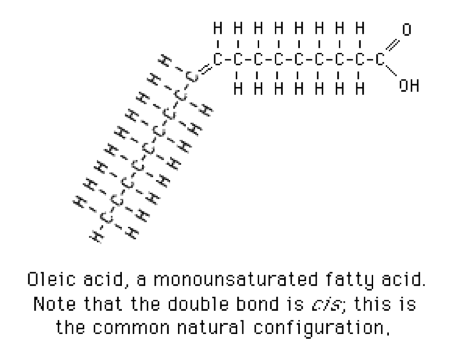
- 1 double bond: monounsaturated fatty acids. Oleic acid is a typical monounsaturated fatty acid (Figure 3).[2]
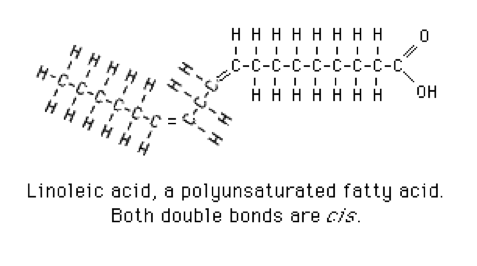
- 2 or more double bonds: polyunsaturated fatty acids. Linoleic acid is a typical polyunsaturated fatty acid (Figure 4).[3]
Figure 4 Linoleic Acid
Saturated fat is a type of fat found in food. For many years, there has been a concern that saturated fats may lead to an increased risk of heart disease; however, there have been studies to the contrary and the literature is far from conclusive. The general assumption is that the less saturated fat the better as far as health is concerned. For the fat manufacturer, however, low saturated fat levels make it difficult to produce oils that will stand up to the high temperatures necessary for processes such as deep-frying. Hydrogenation has been technology’s solution. Hydrogenation will be discussed later in the chapter.
Saturated fat is found in many foods:
- Animal foods (like beef, chicken, lamb, pork, and veal)
- Coconut, palm, and palm kernel oils
- Dairy products (like butter, cheese, and whole milk)
- Lard
- Shortening
Unsaturated fat is also in the foods you eat. Replacing saturated and trans fats (see below) with unsaturated fats has been shown to help lower cholesterol levels and may reduce the risk of heart disease. Unsaturated fat is also a source of omega-3 and omega-6 fatty acids, which are generally referred to as “healthy” fats. Choose foods with unsaturated fat as part of a balanced diet using the U.S. Department of Health and Human Service’s Dietary Guidelines.
Even though unsaturated fat is a “good fat,” having too much in your diet may lead to having too many calories, which can increase your risk of developing obesity, type 2 diabetes, heart disease, and certain types of cancer.
There are two main types of unsaturated fats:
- Monounsaturated fat, which can be found in:
- Avocados
- Nuts and seeds (like cashews, pecans, almonds, and peanuts)
- Vegetable oils (like canola, olive, peanut, safflower, sesame, and sunflower)
- Polyunsaturated fat, which can be found in:
- Fatty fish (like herring, mackerel, salmon, trout and smelt)
- Fish oils
- Nuts and seeds (like cashews, pecans, almonds and peanuts)
- Vegetable oils (like canola, corn, flaxseed, soybean and sunflower)
Hydrogenation
Simply put, hydrogenation is a process of adding hydrogen gas to alter the melting point of the oil or fat. The injected hydrogen bonds with the available carbon, which changes liquid oil into solid fat. This is practical, in that it makes fats versatile. Think of the different temperature conditions within a bakery during which fat must be workable; think of the different climatic conditions encountered in bakeries.
Trans Fat Trans fat is made from a chemical process known as “partial hydrogenation.” This is when liquid oil is made into a solid fat. Like saturated fat, trans fat has been shown to raise LDL or “bad” cholesterol levels, which may in turn increase your risk for heart disease. Unlike saturated fat, trans fat also lowers HDL or “good” cholesterol. A low level of HDL-cholesterol is also a risk factor for heart disease.
Until recently, most of the trans fat found in a typical American diet came from:
- Fried foods (like doughnuts)
- baked goods including cakes, pie crusts, biscuits, frozen pizza, cookies, and crackers
- stick margarine and other spreads
The US Food and Drug Administration (FDA) specifically prescribe what information must be displayed on a label. The trans fat content of food is one piece of core nutrition information that is required to be declared in a nutrition facts table. More information on a nutrition facts table and labeling details can be found in www.fda.gov/food/ingredientsp.../ucm274590.htm
Emulsification (Emulsified Shortenings)
Emulsification is the process by which normally unmixable ingredients (such as oil and water) can be combined into a stable substance. Emulsifiers are substances that can aid in this process. There are natural emulsifiers such as lecithin, found in egg yolks. Emulsifiers are generally made up of monoglycerides and diglycerides and have been added to many hydrogenated fats, improving the fat’s ability to:
- Develop a uniformly fine structure
- Absorb a high percentage of sugar
- Hold in suspension a high percentage of liquid
Emulsified shortenings are ideal for cakes and icings, but they are not suitable for deep-frying.
Stability
Stability refers to the ability of a shortening to have an extended shelf life. It refers especially to deepfrying fats, where a smoke point (see below) of 220°C to 230°C (428°F to 446°F) indicates a fat of high stability.
Smoke Point
The smoke point is the temperature reached when fat first starts to smoke. The smoke point will decline over time as the fat breaks down (see below).
Fat Breakdown
The technical term for fat breakdown is hydrolysis, which is the chemical reaction of a substance with water. In this process, fatty acids are separated from their glycerol molecules and accumulate over time in the fat. When their concentration reaches a certain point, the fat takes on an unpleasant taste, and continued use of the fat will yield a nasty flavor. The moisture, which is at the root of this problem, comes from the product being fried. This is why it is a good reason to turn off the fryer or turn it to “standby” between batches of frying foods such as doughnuts. Another cause of fat breakdown is excessive flour on the product or particles breaking off the product. Attribution
Figure 2. Stearic Acide. Retrieved from http://library.med.utah.edu/NetBioch...Acids/3_3.html ↵
Figure 3 Oleic Acid Retrieved from: http://library.med.utah.edu/NetBioch...Acids/3_3.html ↵
Figure 4 Linoleic Acid Retrieved from: http://library.med.utah.edu/NetBioch...Acids/3_3.html