25: Acid Base
- Page ID
- 143629
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Acid-Base Chemistry
Definitions and Introduction to Lewis Acids and Bases
Lewis Base: electron pair donor
Lewis Acid: electron acceptor
Lewis Acid/Base Complexes (Dative bonds)
-
What structural feature would be required for a Lewis Base?
-
Which of the central atoms is most likely to behave as a Lewis base?
a) BH3 b) BeH2 c) CH4 d) H2O
-
If a Lewis Acid accepts electrons, then it must have a(n) [empty orbital / positive charge / dipole ]. Circle one.
-
Which of these compounds is most likely to behave as a Lewis acid?
a) BH3 b) NH3 c) CH4 d) H2O
-
For the molecules below:
-
Add lone pairs of electrons
-
Circle those that are Lewis acids
-
Underline those that are Lewis Bases
-
Indicate if any of the structures are neither a base or an acid
-
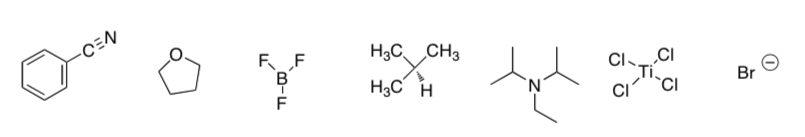
Strengths of Lewis Acids
-
Rank the following Lewis acids in order of increasing acidity and explain your ranking:
Fe+3 Fe+2 Fe+1
-
Conjugated systems can stabilize cations.
-
Draw resonances structures.
-
Rank the following structures in order of increasing acidity:
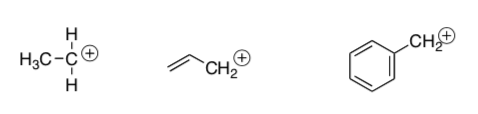
-
-
CH3 groups are electron donating toward cations.
-
Draw dipoles.
-
Rank the following structures in order of increasing acidity:
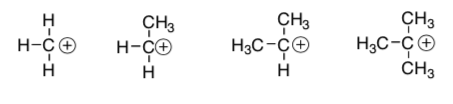
-
MO of Lewis Acid-Lewis Base Reactions
-
Label the Lewis Base and the Lewis Acid in this reaction.
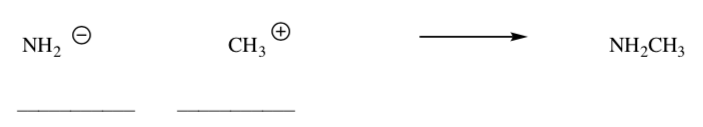
-
In Molecular Orbital Theory, the HOMO (highest occupied orbital) has a pair of electrons to donate so it will be the [ base / acid ]. Circle one.
-
In Molecular Orbital Theory, the LUMO (lowest unoccupied orbital) will be the[ base / acid ] because it is empty.
The orbitals for these two reactants are shown here:
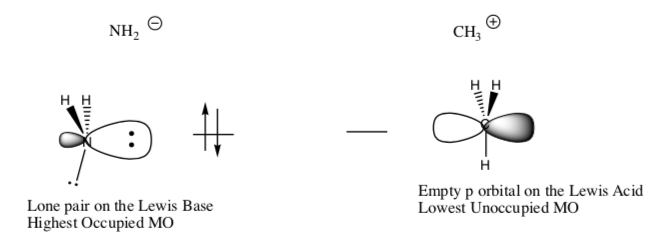
-
When two p orbitals are overlapped head to head, they can overlap in-phase or out- of-phase. Label these two possible orbital combinations as π, π*, σ or σ*
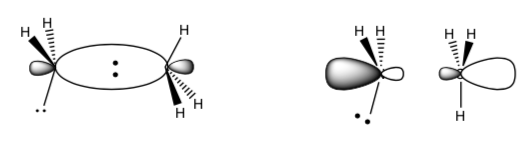
-
Draw the MO diagram for the formation of these two orbitals with the correct number of electrons.
Curved Arrow Notation for Lewis Acid-Lewis Base Reactions
Rules for Arrows:
Arrows always begin at the source of electrons (nucleophile or Lewis Base).
Arrows always end at the electrophilic (electron deficient or Lewis Acid).

This accounting of bonds formed and broken is called a reaction mechanism.
-
Predict the products for the following Lewis Acid-Lewis Base Reactions.
-
Use curved arrows to show the mechanism of the following reactions).
-
Label the Nucleophile (Lewis Base) and the Electrophile (Lewis Acid) in the starting materials.
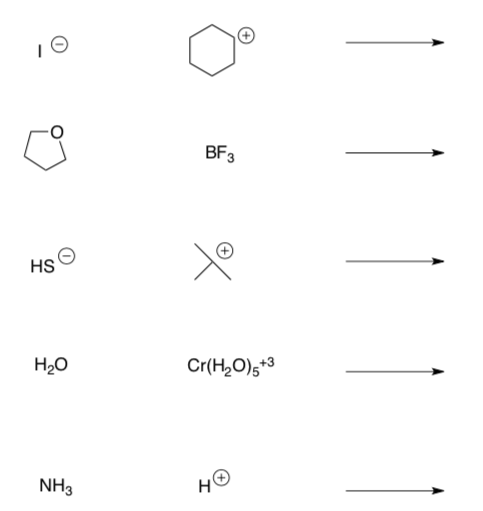
Two Arrow Lewis Acid-Lewis Base Reactions
Sometimes there are two arrows for these Lewis Acid:Lewis Base reactions.
-
Use curved arrows to show the mechanism of the following reactions.
-
Propose a reason why these reactions need a second arrow.
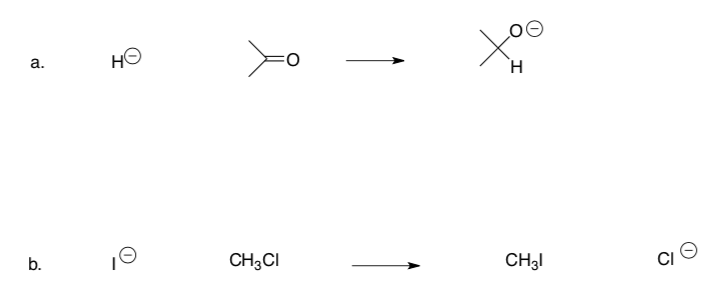
Introduction to Bronsted Acids and Bases
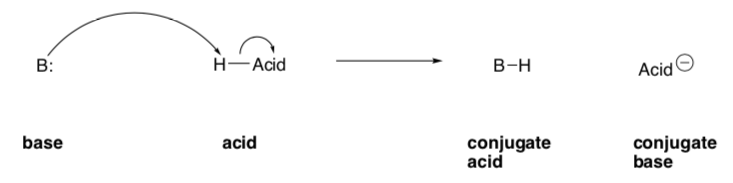
Bronsted-Lowry Acids: proton donor
Bronsted-Lowry Bases: proton acceptor
In an acid-base reaction, an acid plus a base reacts to form a conjugate base plus a conjugate acid:
-
What is the structural requirement for a molecule to be a proton acceptor (Bronsted Base)?
-
What is the structural requirement for a molecule to be a proton donor (Bronsted Acid)?
The acid and conjugate base as well as the base and conjugate acid are known as conjugate pairs. The acid and base are found on the reactant side of the equation; the conjugate acid and conjugate base are the products.
-
The Bronsted __________ gains a proton (H+), so the compound that has one more hydrogen ion is its conjugate ____________.
-
The Bronsted ___________ loses a proton (H+), so the compound that has one less hydrogen ion is its conjugate ____________.
Curved Arrow Notation for Bronsted Acid-Base Reactions
(Also known as Proton Transfers)
For proton transfer reactions, there are always two arrows: 1) formation of base-proton bond and 2) breaking the bond from the proton to the acid.

-
Use curved arrows to show the proton transfer mechanism of the following reactions. The proton in bold will be removed.
-
Predict the product for the following reactions.
Compounds that can be Both Acids and Bases
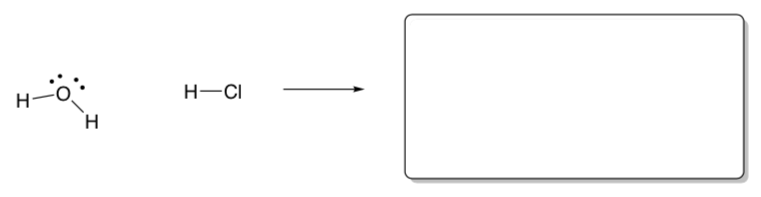
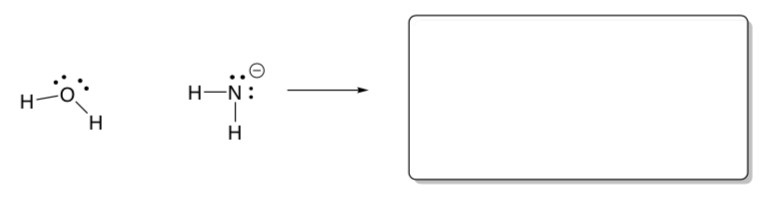
An amphiprotic molecule can either donate or accept a proton, thus acting either as an acid or a base.
Water is a classic example of an amphiprotic molecule.
-
Draw a curved arrow mechanism for water acting as a base.
-
Draw a curved arrow mechanism for water acting as an acid.
MO of Proton Transfer Reactions
A common proton transfer reaction:

Molecular Orbital representation of the same reaction:
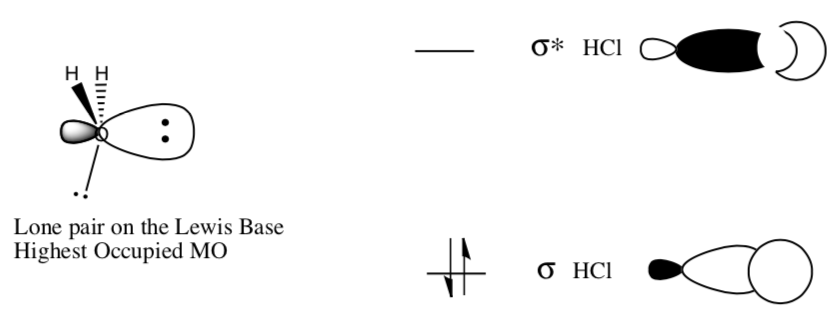
An electron pair from water is ready to react with the HCl.
-
Which orbital is willing to donate electrons?
-
Which orbital can accept electrons?
-
Is the water able to form an in-phase (constructive interference) overlap with the H+?
-
Indicate the two lobes that will overlap to form the new bond.
-
Draw a molecular orbital cartoon of the overlap of the base with H+ (the product).
Summary of Lewis Acids and Bases
-
Complete these definitions:
Lewis Acid: ________________
Lewis Base: ________________
Bronsted-Lowry Acids: ________________
Bronsted-Lowry Bases: ________________ -
What is the structural requirement for a molecule to be a Bronsted Base? Lewis Base?
-
What is the structural requirement for a molecule to be a Bronsted Acid?
-
What is the structural requirement for a molecule to be a Lewis Acid?
-
Name three or more categories of molecules that can act as Lewis Acids.
-
For a Bronsted acid/base pair, answer the following questions:
-
How is the formula of an acid different from that of the conjugate base?
-
How is the formula of a base different from that of the conjugate acid?
-
-
In curved arrow mechanisms:
-
The arrows move from ____________ to _____________.
-
The arrows represent [movement of atoms / movement of electrons ]. Circle one.
-
Structural Factors Controlling Bronsted Acidity:
Which Proton? How Easily?
pKa: An experimental measurement that indicates how easily a Bronsted Acid will give up its proton.
High pKa = weak acid (won’t give its proton away)
Low pKa = strong acid (dissociates easily)
To determine the acidity of a compound, look at the stability of the conjugate base.
The more stable the conjugate base, the more likely the compound will be willing to give away the H+.
-
A strong acid has a weak conjugate base
-
A weak acid has a strong conjugate base
-
Which of these compounds is more acidic? Draw the conjugate base for each. What factor controls the trend in acidity among these three molecules?
HF H2O NH3 CH4
pKa 3 16 36 50
-
Same question, new molecules.
HF HCl HBr HI
pKa 3 -7 -9 -10
-
Same questions, new molecules.

-
Same question, new molecules.
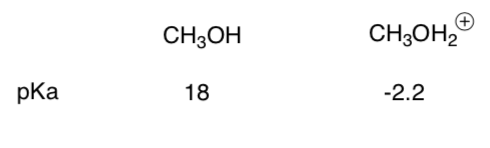
-
Same question, new molecules.
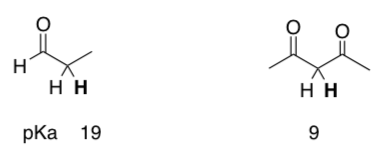
-
Same question, new molecules.
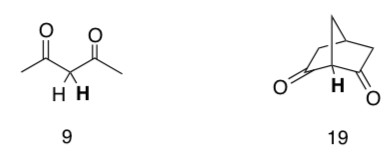
-
Same questions, new molecules.
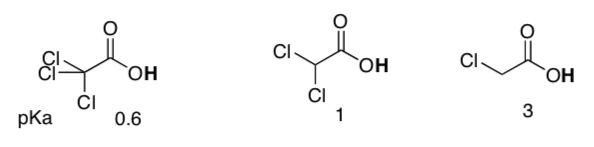
-
Same question, new molecules.
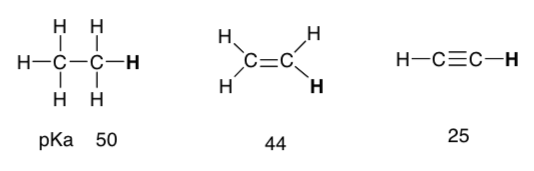
Summary of Structural Features that influence Acidity/Basicity:
pKa: An experimental measurement that indicates how easily a Bronsted Acid will give up its proton.
High pKa = ____________ (strong or weak acid)?
Low pKa = ____________ (strong or weak acid)?
Explain each of the structural features that effect pKa using an example:
-
Electronegativity
-
Atomic size (polarizability)
-
Delocalization (resonance)
-
Hybridization
-
Inductive (dipole)
-
Charge
Additional Problems
-
A lower pKa indicates that the compound is a (weaker or stronger) ___________ acid.
-
A higher pKa indicates that the conjugate base of the listed compound is a (weaker or stronger) ___________ base.
-
Metals act as:
-
Lewis acids
-
Lewis bases
-
Bronsted acids
-
Bronsted bases
-
-
A very strong base will react with:
-
Weak acid
-
Strongacid
-
Any acid
-
Weakbases
-
-
Bronsted bases and Lewis bases and ligands all have a similar structural feature that is required for activity: __________________
-
Select all of the functional groups below that would be good Lewis Bases
-
alkene
-
alcohol
-
alkane
-
aromaticring
-
amine
-
ketone
-
thiol
-
-
On each of the following compounds, identify the most acidic proton. Think about polarizability, electronegativity, resonance, etc.
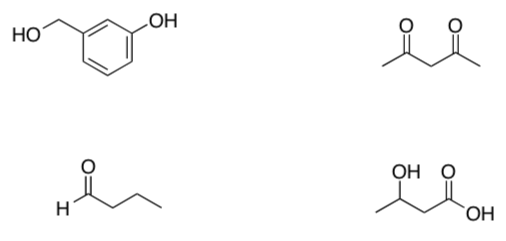
-
Circle the most acidic proton in the following compounds:
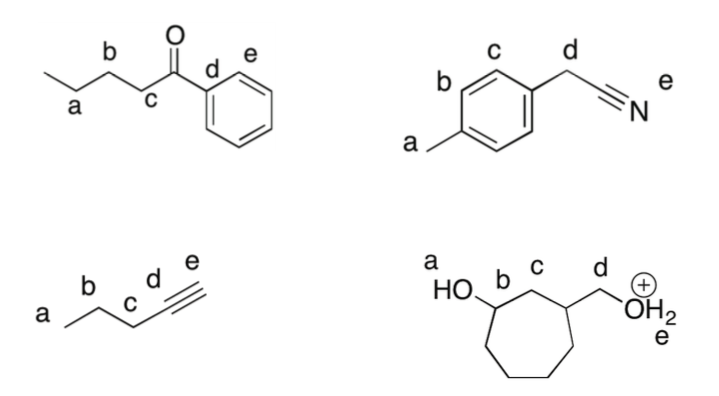
-
In each pair of compounds, choose the stronger acid. Identify the factor that explains the stability of the conjugate base (e.g. polarizability, electronegativity, etc.)
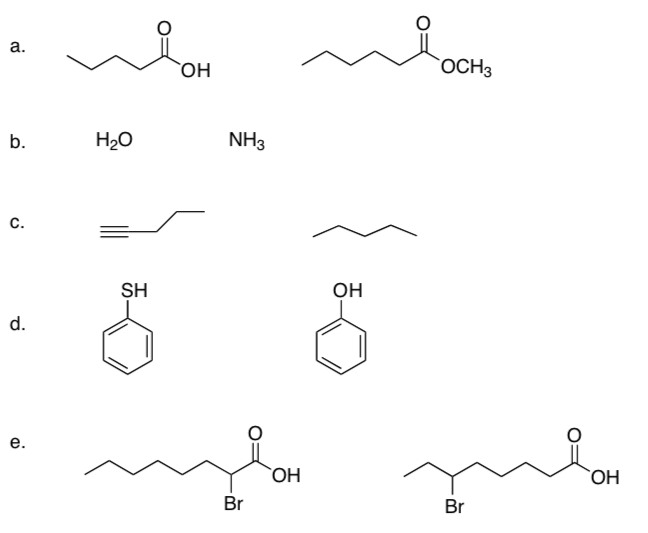
-
Explain why H2SO4 has a pKa of 0, while HSO4- has a pKa of 1.9.
-
Explain why the 1,4 disubstituted isomer is more acidic than the 1,3 disubstituted isomer. Draw the conjugate bases and several resonance structures of each.
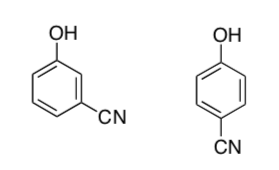
-
pKa trends for allylic and propargylic (a to an alkyne) systems:
-
Draw the conjugate bases for each acid below
-
Using their conjugate bases, explain the pKa differences using Hückel MO diagrams.
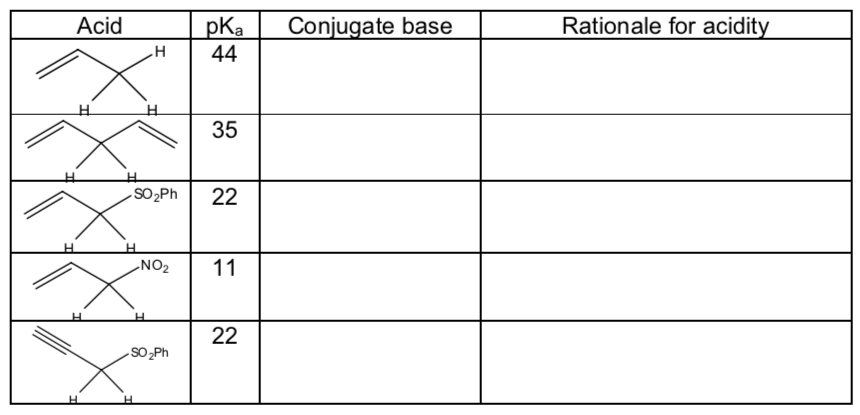
-
-
pKa trends for carbonyl systems:
-
Draw the conjugate bases for each acid below.
-
Using their conjugate bases, explain the pKa differences using Hückel MO diagrams.
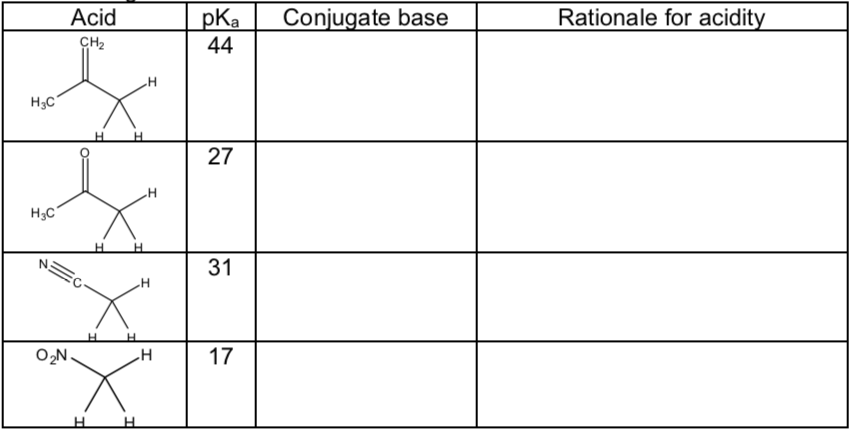
-
-
Chlorocyclopentadiene does not ionize easily in solution. It will ionize only when treated with a very strong Lewis acid such as antimony pentafluoride (SbF5). The cation produced is found by electron paramagnetic resonance (epr) studies to have two unpaired electrons.
-
Fill in the missing structure.
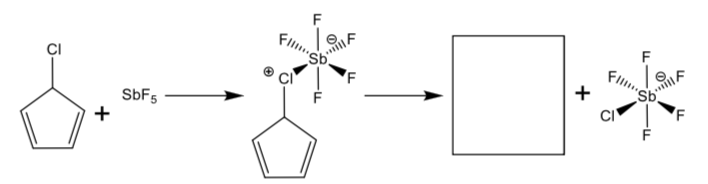
-
Explain why the cation has unpaired electrons (in contrast to what you drew in the box above). HINT: Draw the MO diagram.
-
Explain why this cation is difficult to form.
-
Structural Factors Controlling Basicity
Try it in reverse. The same structural features control basicity.
To determine the basicity of a compound, look at the stability of the base.
The more stable base is less likely the compound to give away a lone pair.
-
Knowing the relationship between basicity and acidity, order the following ions by increasing basicity (weakest to strongest).
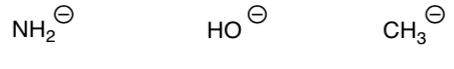
-
Looking at a pKa Table, list 4-5 very strong bases.
Additional Problems
-
In each pair of compounds, choose the stronger base:
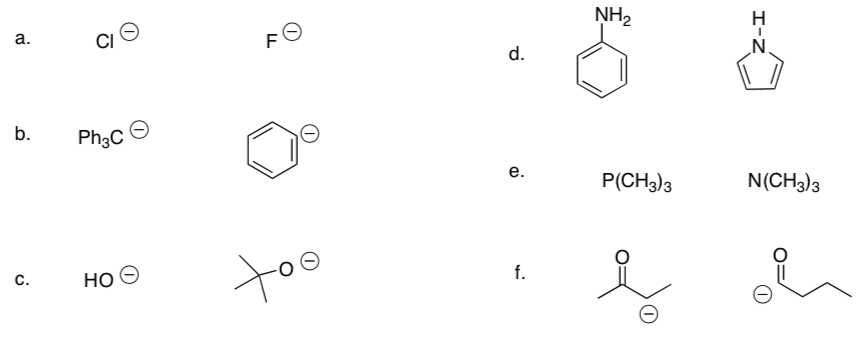
Additional Problems
-
Cyclopentadiene(shown below)is an instructive example of acyclic, partially conjugated compound. Its pKa can be determined by treating cyclopentadiene with varying amounts of NaOH in water and measuring the absorbance of the cyclopentadienyl anion by UV-Visible spectroscopy.
-
Show an equation, with curved arrows, for the reaction of cyclopentadiene with NaOH.
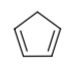
-
The resulting pKa, about 16, is extraordinarily low for a hydrocarbon (it is quite similar to that of water). Explain why this is unusual...
-
In terms of what you know about a C-H bond vs. an O-H bond.
-
In terms of what you know about a carbon anion vs. an oxygen anion.
-
-
Explain, with the aid of a Hückel MO diagram, why the cyclopentadienyl anion is very stable.
-
Table 1. pKa values of representative functional groups

Equilibrium in Proton Transfer Reactions: Who gets the Proton?
Analogy:
-
If you give one toy to two children, what will happen? Just in case you don’t have siblings and you haven’t ever babysat, the answer is the two children will fight over the toy.
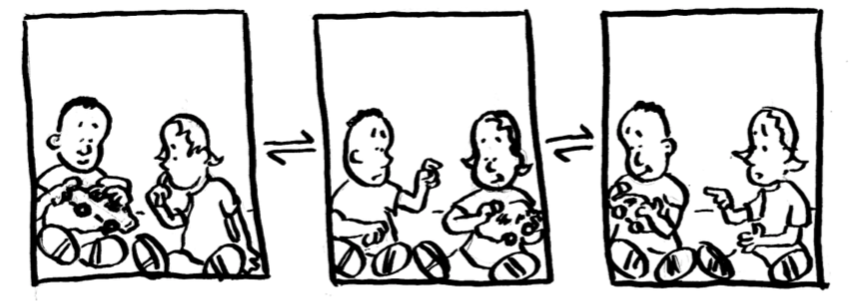
-
If the children are both two year olds, will one of the children be more likely to get the toy? Describe what happens to the toy (as shown in the pictures above).
-
If one of the children is 2 years old and one of the children is 10 years old, who will have the toy the majority of the time?
-
If the children’s’ ages are pKas, explain what happens to acidity as the pka increases? Is a compound with a high pKa likely to give away its toy (ie its proton)?
Brief Quantitation
For a general chemical reaction, K is defined as the ratio of products: reactants:
reactants products
$$
\mathrm{K}=\frac{|\text { products }|}{|\text { reactants }|}
$$
-
If reaction favors the products, K (the equilibrium constant) will be:
-
large (>1)
-
small (<1)
-
about 1
-
-
If reaction favors the starting material (ie not very reactive), K will be:
-
large (>1)
-
small (<1)
-
about 1
-
-
If the reaction goes about half way to completion, K will be:
-
large (>1)
-
small (<1)
-
about 1
-
-
The pKa of water is 16. What does this number say about the equilibrium constant in the ionization reaction? (K = 10-pka) Is it greater or less than 1? What does this mean?

Using pKa to Predict Reaction Direction
To determine whether reactants or products are favored in an acid base reaction, follow these steps.
-
Determine the molecule that will act as the acid on both sides of the equation.
-
Locate most acidic proton and look up the pKa for each acid using a table.
-
The reaction will favor the side of the reaction where the molecule with the highest pKa is protonated.
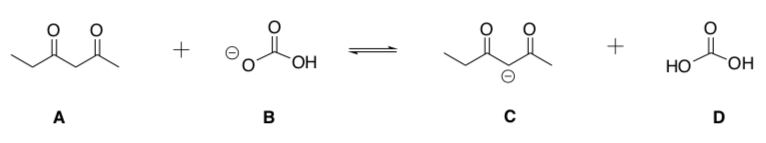
-
For the reaction above, which two compounds are the acids?
-
Estimate the pKa for each of these two compounds are the acids?
-
Are products or reactants favored? Is the pKa larger for the acid on the left or right side of the reaction?
-
Using a pKa Table, predict the equilibrium constant for this reaction:
-
would be large (>1)
-
would be small (<1)
-
would be about 1
-
is impossible to estimate with the information given
-
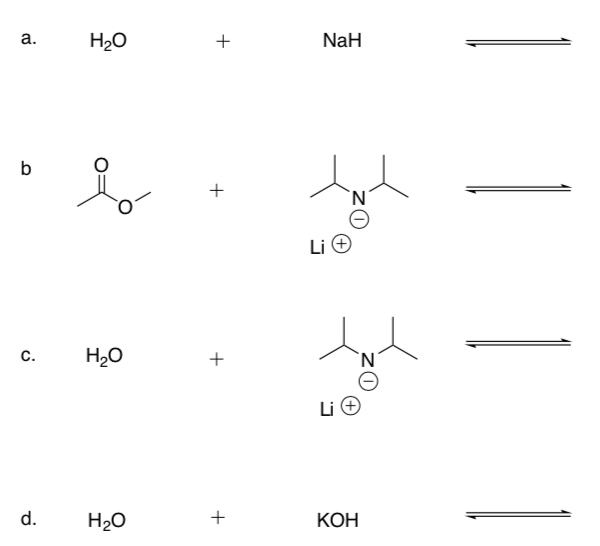
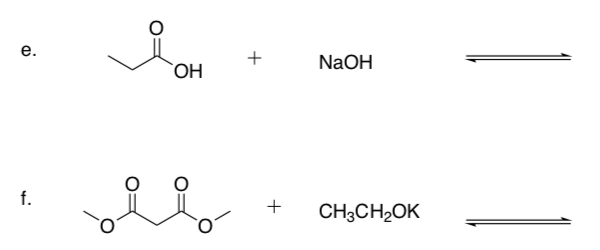
Practice Predicting Reaction Direction
-
Draw the mechanism for each base reacting with a protic (Bronsted) acid.
-
Show the products of these acid-base reactions.
-
Use a pKa Table to predict whether the reactants or products will be favored.
Note
Li+, Na+ and K+ tend to be ‘spectator ions’ to balance charge and do not participate in the reaction.
Equilibrium of Acids and Bases in Aqueous Solutions
When a strong acid is added to water, the acid is fully dissociated. In other words, the H2O acts as a Lewis base to take the H+ (proton) from the acid.
-
Draw an arrow mechanism for this reaction:

Another way to picture this:
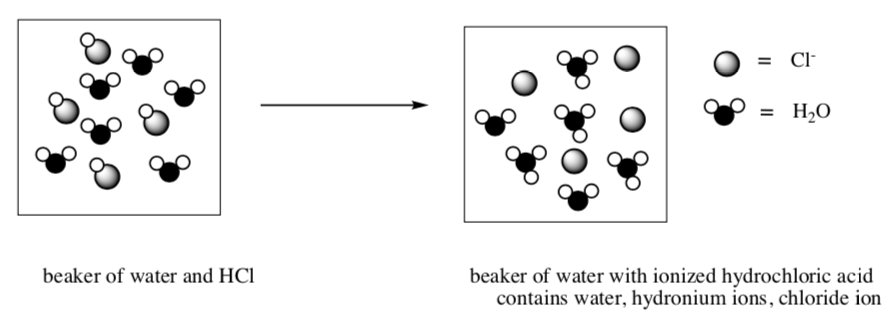
When a weak acid is added to water, the acid does not fully dissociate.
- Draw an arrow mechanism and product for this reaction:
- Draw a cartoon to represent the amounts of products formed in this reaction:
When a strong base is added to water, the base removes the H+ (proton) from the water. In other words, it becomes protonated and hydroxide ion forms.
- Draw an arrow mechanism for this reaction:
- Draw a cartoon to represent the amounts of products formed in this reaction:
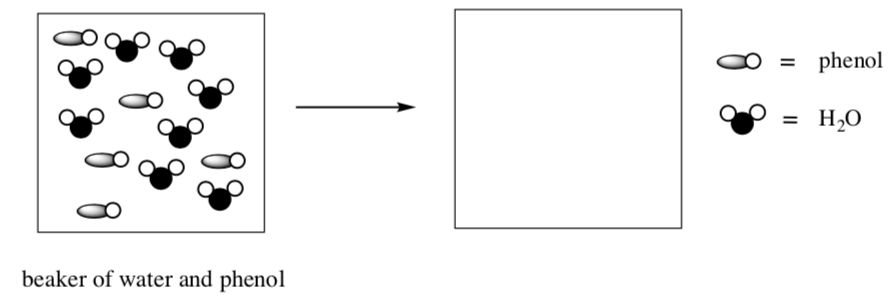
When a weak base is added to water, the base does not fully dissociate.
-
Draw an arrow mechanism and product for this reaction:
- Draw a cartoon to represent the amounts of products formed in this reaction:
Summary of Leveling Effects
Based on previous exercises on dissociation of acids and bases in aqueous solutions:
-
What is the strongest acid that remains in solution, when a strong acid is added to water?
-
What is the strongest base that remains in solution, when a strong base is added to water?
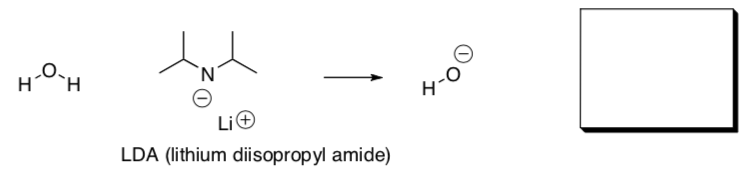
Stronger Bases such as BuLi, NaH, LDA can exist in organic solvents but are quickly protonated in water. In fact, this is so exothermic that it can be explosive.
Acid-Base Extractions
Acid-base extraction is a procedure to separate an acids and/or bases from a mixture based on the change in solubility of the conjugate base (or acid).
For example, if you have a mixture of benzoic acid and propylbenzene:
- These compounds contain [mostly polar bonds / mostly nonpolar bonds].
And then you add this mixture to a beaker with ether and water.
- Draw a cartoon showing which solvent these compounds dissolved in.
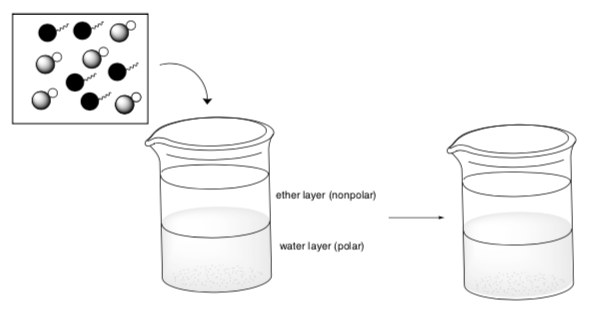
-
Which compound is acidic? benzoic acid OR propylbenzene
-
Draw the reaction of this compound with NaOH.
-
Charged compounds are soluble in water. Draw a cartoon representing which layer each compound is in now.

-
If you separate the two liquid layers, have you separated benzoic acid and propyl benzene?
-
How could you convert the conjugate base of benzoic acid back to benzoic acid?
Acid-Base Extractions (cont)
Acid-base extractions can even separate two acids if they have different pKa values.
-
What are the pKa values for the following two compounds?

-
Based on pKa values, would the reaction of benzoic acid and NaOH favor reactions or products?
-
Based on pKa values, would the reaction of benzoic acid and NaHCO3 favor reactions or products?
-
Based on pKa values, would the reaction of phenol and NaOH favor reactions or products?
-
Based on pKa values, would the reaction of phenol and NaHCO3 favor reactions or products?
Using an understanding of differential reactivity, the mixture can be separated by using a weaker base to react only with the stronger acid.
- Complete the following scheme.
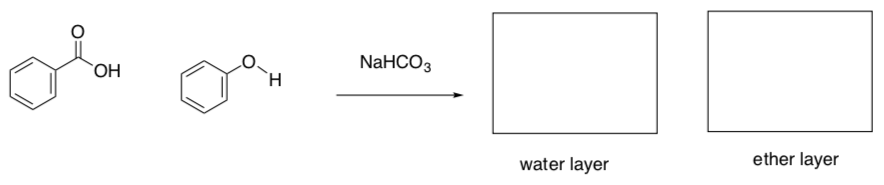
Acid-Base Extraction
An organic chemistry student was given a mixture of four compounds for his lab practical. He decided to use an acid base extraction. Label each compound as neutral, acidic or basic.
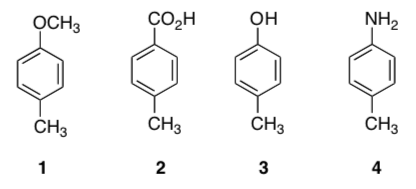
This student proposed the following separation outlined in the flow chart below.
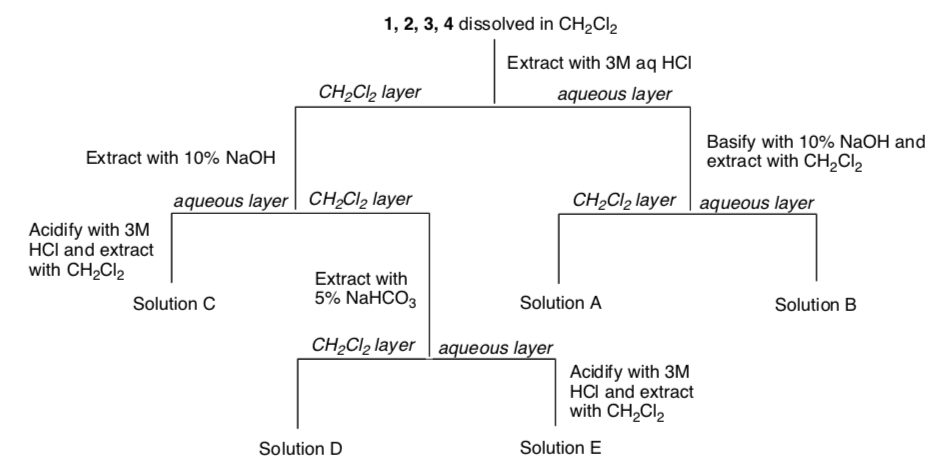
-
Indicate what is in each solution:
Solution A
Solution B
Solution C
Solution D
Solution E
-
There is a problem with the students proposed separation scheme. What is the problem and how could it be fixed?
Additional Problems
-
There is a very high market value on converting the following natural compound to its conjugate base (the “free base”).
-
How would you carry out this reaction using acid-base extraction?
-
Be specific about solvents and strength of base and solubilities.
-
Draw the product.
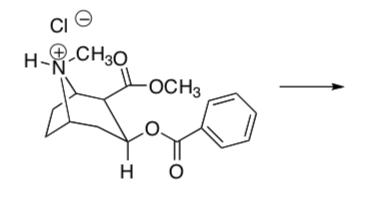
-
-
A student has a mixture of phenol and benzoic acid (C6H5CO2H). The compounds could be separated via an acid/base extraction using what base?
a) NaOH b) NaH c) NaHCO3 d) NaNH2
-
A student dissolved a mixture of benzoicacid(C6H5CO2H), phenol(C6H5OH) and 1,4 dimethoxybenzene (CH3OC6H4OCH3) in t-butyl methyl ether. She extracted the solution with 5% aqueous sodium bicarbonate, and labeled the extract A. She then extracted the ether solution with 1 M aqueous sodium hydroxide, and labeled that extract B. She added 12 M aqueous HCl to these two extracts, producing a white precipitate in each. The precipitate obtained from solution B is probably...
a) benzoic acid b) phenol c) dimethoxybenzene d) t-butyl methyl ether
-
When a metal ion dissolves in water, the cation is hydrated. Many ions are surrounded by 6 water molecules, forming hexahydrates.
-
With sodium ion as the metal, show the hydration process using a curved arrow mechanism of the metal ions binding waters.
-
Identify the Lewis acid and the Lewis base.
Once the hydration reaction is complete, the complex can undergo additional acid/base reactions, as shown below:
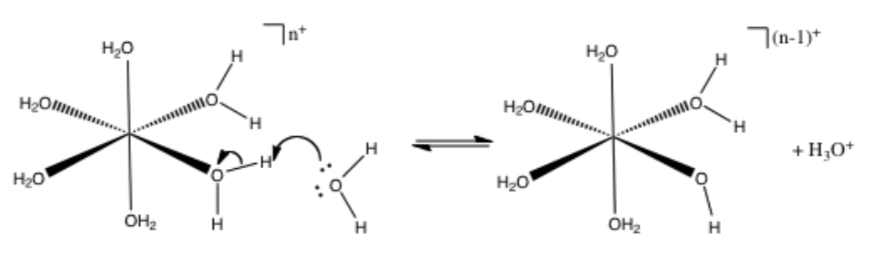
-
Identify the Lewis acid, Lewis base, the conjugate acid and the conjugate base in the reaction above.
The pKa value for [Fe(H2O)6]2+ is 9.5; the pKa value for [Fe(H2O)6]3+ is 2.2.
-
Which is the stronger acid?
-
Which is the stronger conjugate base, [Fe(H2O)5(OH)]+ or [Fe(H2O)5(OH)]2+?
-
Explain how the charge of the central ion affects the strength of the base?
When FeCl3 is added to water, Fe(OH)3 is formed and quickly precipitates.
-
Write a mechanism for this reaction.
-
If HCl is added, no ppt is formed. How does the presence of HCl affect the equilibrium of the reaction above?
-
Given your understanding of metal ions forming hexahydrates from the previous problem, which of these is a stronger acid?
Cr2+(aq) or Cr3+(aq)
-
Acid Base Chemistry of Amino Acids
Biological amino acids, such as alanine, contain both an acidic functional group and a basic functional group.
-
Circle the basic group.
-
Place a box around the acidic group.
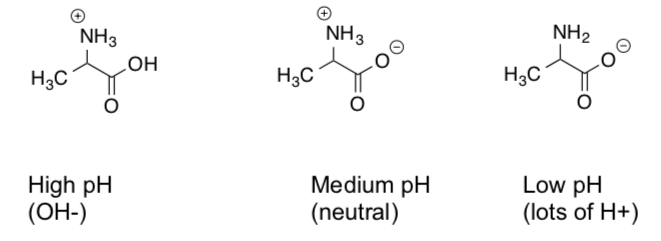
Alanine can exist in different protonation states
- Match the protonation state with the pH of the solution.
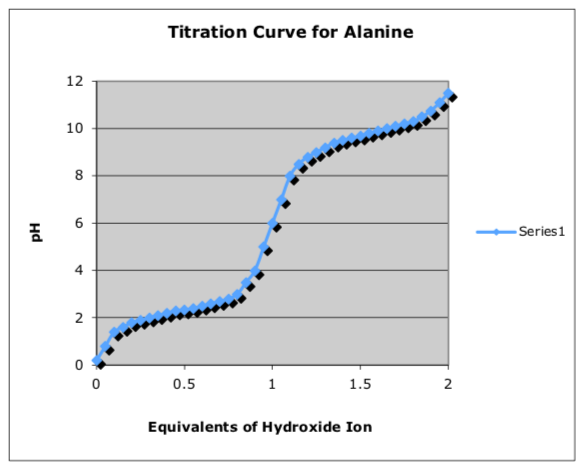
Alanine has "inflection points" in its titration curve -- points where the increase in pH flattens out due to an acid-base reaction -- at pH = 2.34 and 9.69.
- Match the protonation state with the portion of the graph.
"Basic" amino acids, such as lysine, display a third inflection point due to an additional acid-base reaction.
-
Draw the additional acid-base reactions for these basic amino acids.
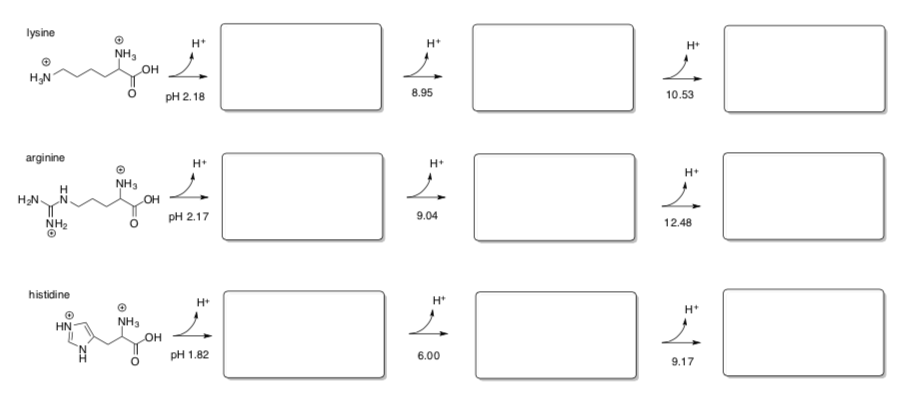
- Explain the differences in pH values at which these additional reactions occur in lysine, arginine and histidine.
One of the simplest tasks an amino acid can perform is to provide protons when they are needed and also to remove protons when necessary.
-
Show how histidine at pH 6 could perform these two proton-shuttling tasks.
Aspartic and glutamic acid also have a third inflection point.
- Draw the protonation states at the different pH conditions.
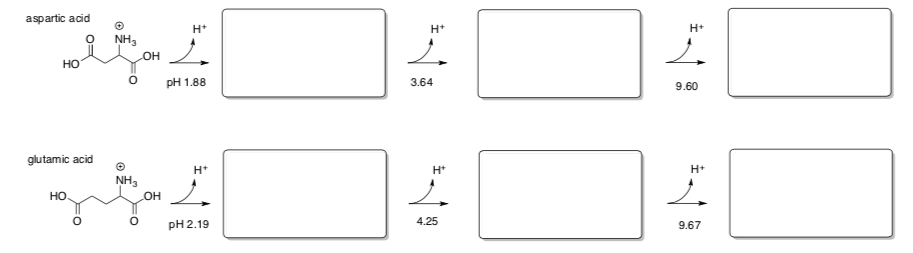
- Draw a titration curve for one of these amino acids.
-
Explain the differences in pH values at which these additional reactions occur in aspartic and glutamic acid vs lysine.
A third acid-base reaction also occurs in cysteine and tyrosine, but not in serine.
- Show the additional reactions that occur in cysteine and tyrosine.
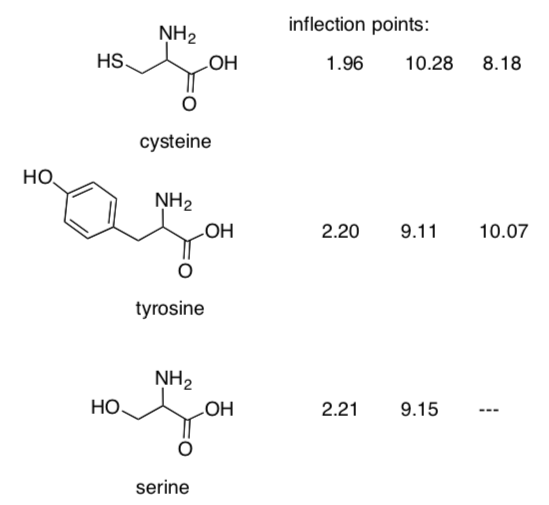
-
Explain why additional acid-base reactions occur in cysteine and tyrosine, but not in serine.
In a process called electrophoresis, different amino acids can be separated from each other by putting the amino acid mixture on a gel and connecting a positive electrode to one end of the gel and a negative electrode to the other end.
-
At pH 6, show what would happen to a mixture of lysine, alanine and aspartic acid in electrophoresis.

