10: Lewis Structures
- Page ID
- 142081
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Lewis Structures and Geometries - Introduction to Main Group (p block) Bonding
Steps for Drawing Lewis Dot Structures
-
Find total number of electrons: valence electrons for all the atoms and adjust for charge.
-
Determine the connectivity. Often this will be given to you. If not, see IM4 in theStructure and Reactivity text.
-
Fill in the remaining electrons to attempt to give all atoms an octet.
-
If any atoms don’t have octets, make double or triple bonds.
-
Leftover electrons are placed on atoms with d orbitals.
Remember that elements in row 3 or below in the periodic table (S, P, etc) can have more than 8 electrons around them. Some elements commonly form compounds with less than 8 (B, Be, Al and obviously H). C, N, O and F ALWAYS obey the octet rule!
-
Calculate Formal Charge on each atom.
# Valence electrons – 1⁄2 # bonding electrons – # non-bonding electrons = F.C.
-
Sometimes, there is more than one way to draw a compound. Formal charge can help to decide which structure is “best”.
Drawing Lewis Structures
- Using the rules from the previous page, complete the Lewis structures for the following compounds:
-
CH4
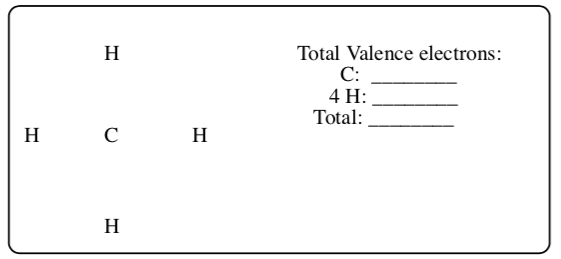
-
NH3
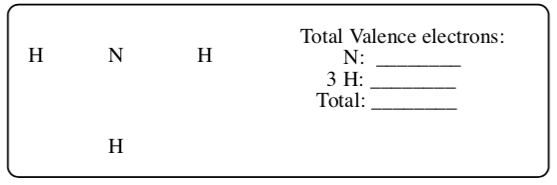
-
PCl3
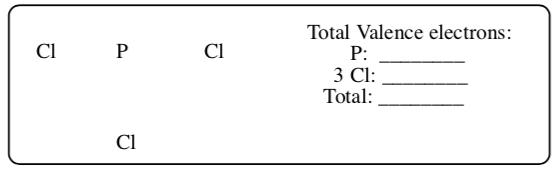
-
H2O

-
BF3

Double bonds to Provide Octets
When there are not enough electrons to give each atom have an octet, you can form a double bond to give both atoms an octet.
- Complete the Lewis structures for the following compounds:
-
CH3(CO)CH3
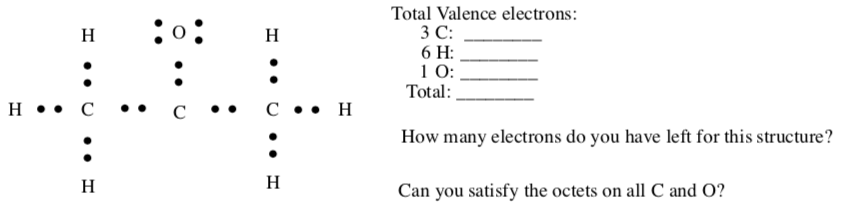
-
CH2CH2
Total Valence electrons:
-
CHCH
Total Valence electrons:
-
HCN
Total Valence electrons:
-
CH3CO2H
Total Valence electrons:
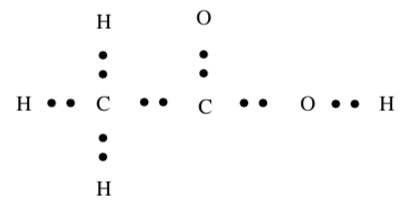
Formal Charge in Lewis Structures
-
Complete the Lewis structures for the following compounds.
-
Calculate the formal change on each atom.
Formal Charge = # Valence electrons – 1⁄2 # bonding electrons – # non-bonding electrons
-
CO
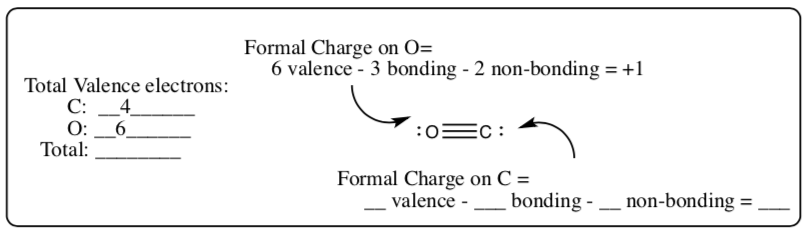
-
HN3 (H-N-N-N)
-
[CH3-O]-
-
[O-N-O]-
-
[H3O]+
-
H2SO4
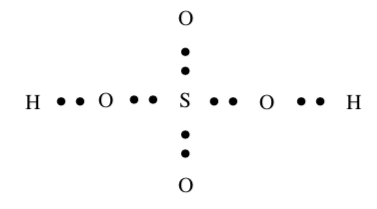
Lewis Structures that exceed the octet rule
Atoms that are larger than neon can exceed the octet rule.
-
Complete the Lewis structures for the following compounds:
-
I3- (I-I-I)
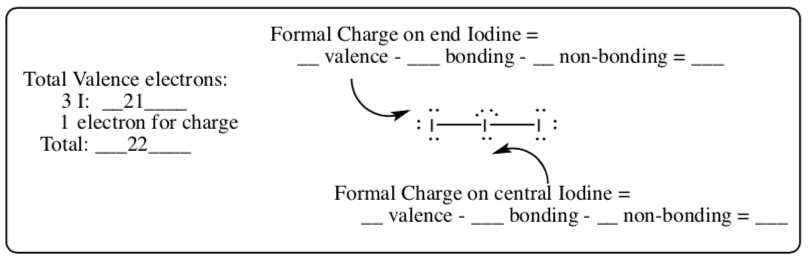
-
ICl4- (Iodine is the central atom)
-
H3PO4 (Phosphorus is the central atom; H are bonded to oxygens)
-
SF4
-
SF6
Lewis Structures of Polyatomic Ions
-
Complete the Lewis structures for salts of polyatomic ions. Assume that the alkali earth metals and the alkali metals are cations. Draw the Lewis structure for the molecular structure.
-
NaNO2
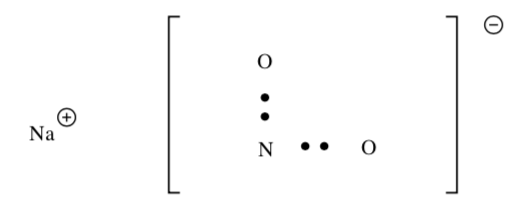
-
KNO3
-
LiAlH4
-
K2HPO4
More Practice with Formal Charge on Lewis Structures
-
This formula will allow you to determine the formal charge on any atom.
__________ = # valence electrons – 1⁄2 # bonding electrons – # non-bonding electrons
-
Assign formal charges on the following compounds.
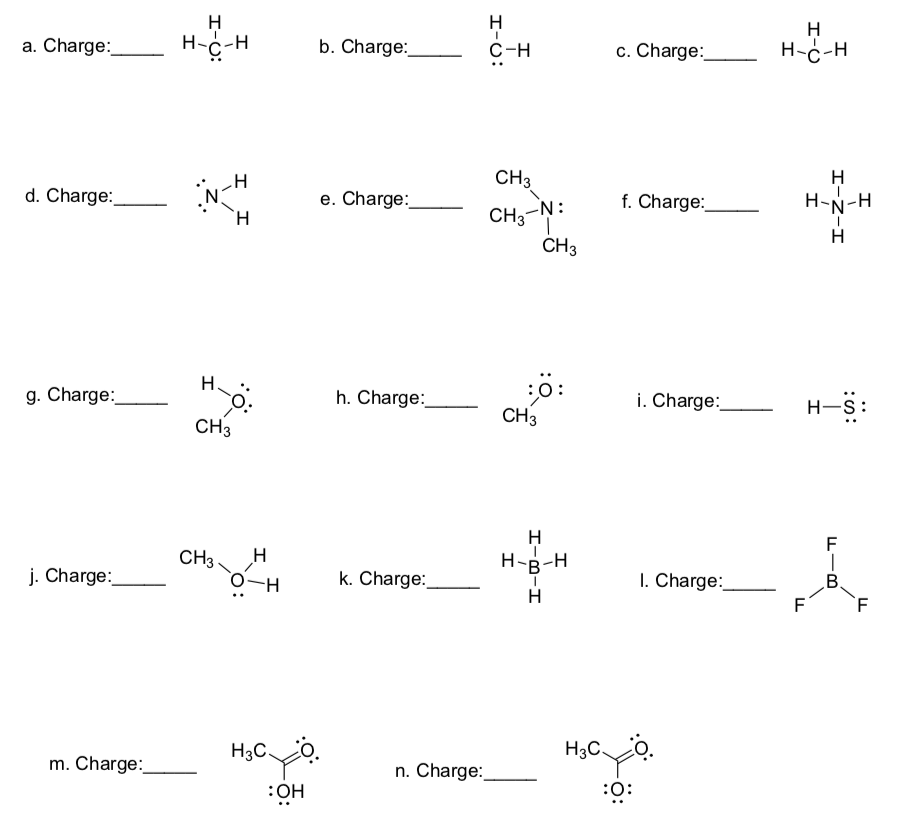
-
-
The same formula will allow you to determine the number of non-bonding electrons if you have the formal charge
Formal charge = # Valence electrons – 1⁄2 # bonding electrons – _____________.
-
Draw in any missing lone pairs on the structures below.
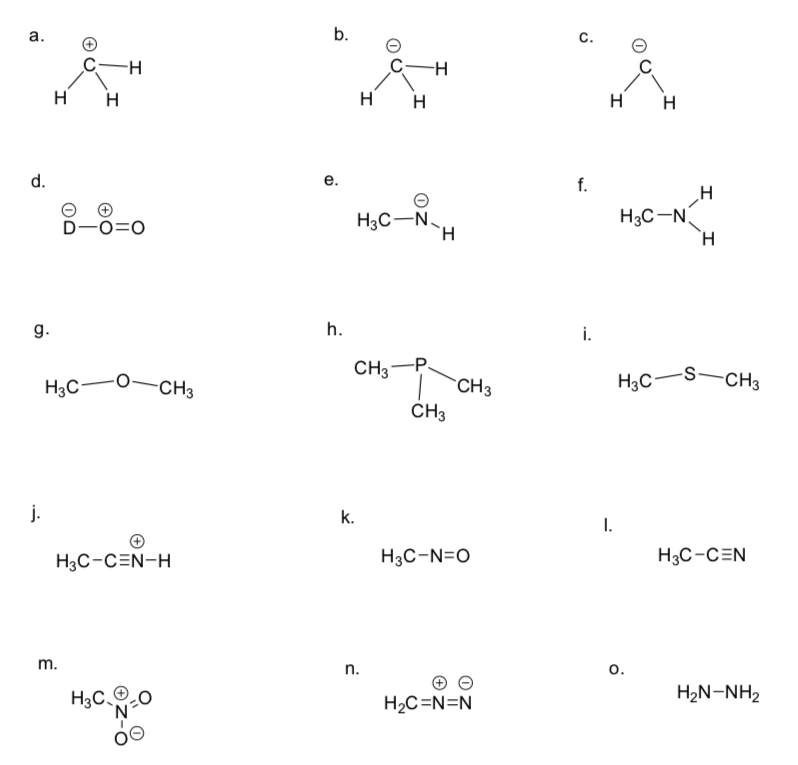
-
-
Look for Patterns in Main Group Bonding and Formal Charges.
In many main group compounds, you can do a quick spot check to see if there will be a formal charge because the bonding in neutral compounds follows a pattern.
-
Complete the following table for Neutral Compounds:
Groups Group 3A (eg BF3) Group 4A (eg CF4) Group 5A (eg PCl3) Group 6A (eg H2O) Group 7A (eg HBr) number of bonds 3 4 number of lone pairs 0 1
Bonding in charged compounds follows a pattern. NOTE: This doesn’t work for compounds with expanded octets!
-
Complete the following table for Anionic Compounds (-1 charge):
Groups
Group 3A (eg BF4)
Group 4A (eg CBr3-)
Group 5A (eg NH2-)
Group 6A (eg HS-)
Group 7A (eg Cl-)
number of bonds
4 3 number of lone pairs
0 1
-
Molecular Geometry
Lewis Structures can be used to predict geometry.
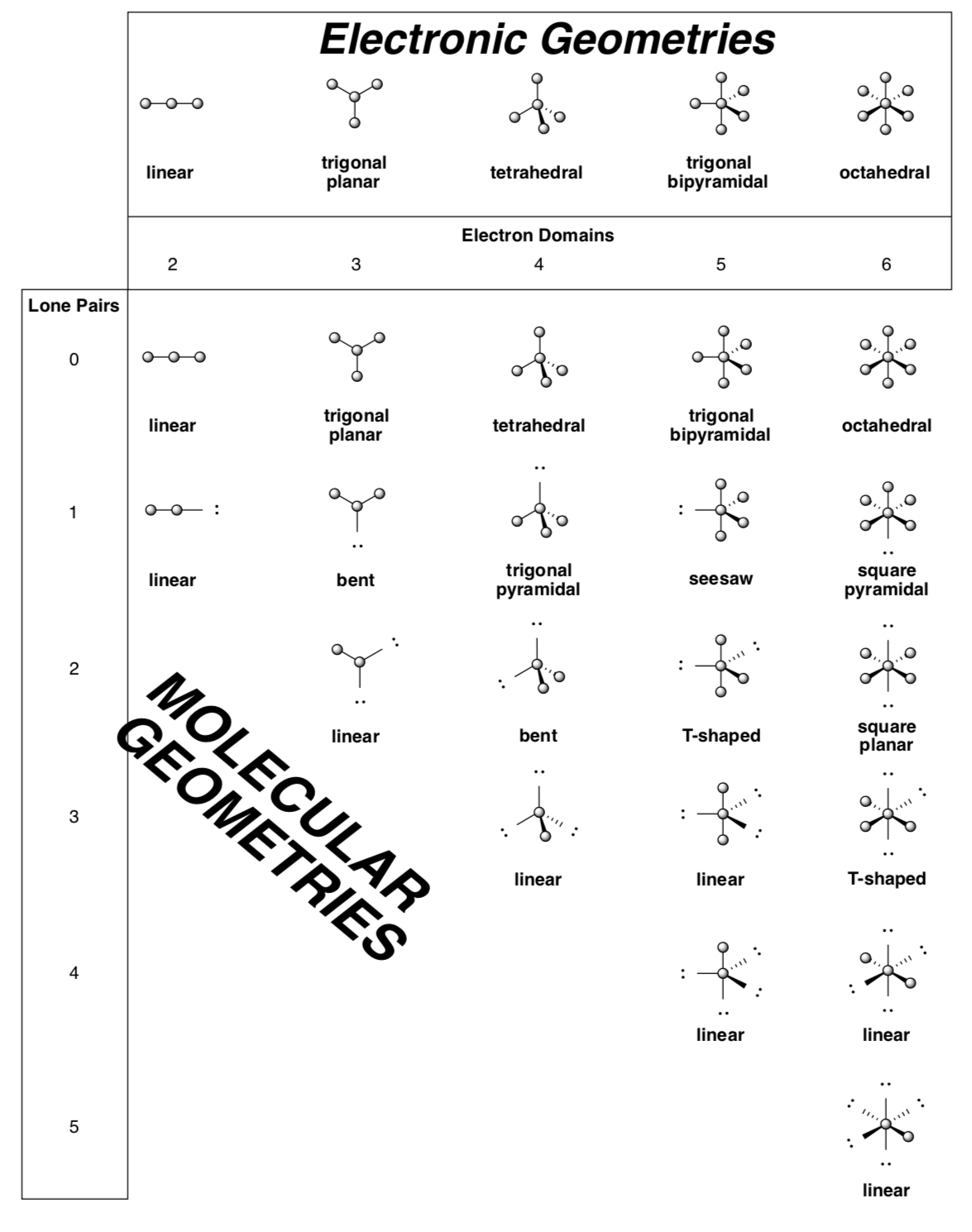
Geometry of Lewis Structures
-
Fill in the blanks on the table below

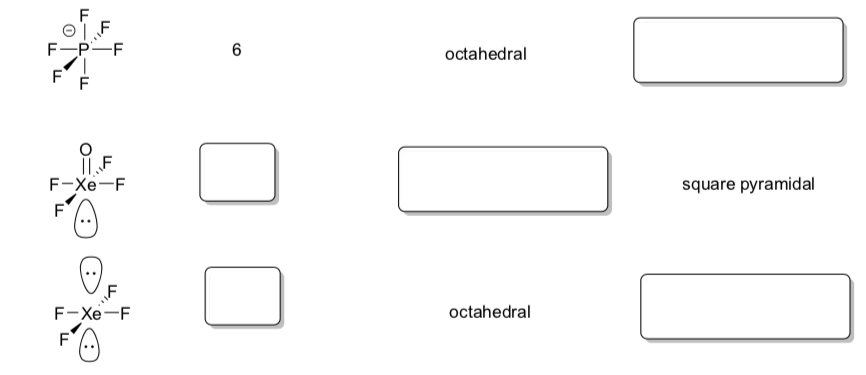
More Practice with Geometry of Lewis Structure
- For each of the molecules or ions in this exercise,
-
Count the number of sites of electron density to determine the electronic geometry.
-
Draw a perspective drawing of the molecule. Use the following methods to indicate bonds projecting out or in the plane of the molecule:
bond projects out of the plane of the molecule

bond projects into the plane of the molecule:

-
Name the molecular geometry (the geometry of the structure neglecting the lone pairs).
-
Compound Lewis Structure Electronic Geometry Molecular Geometry
-
CH4
-
NH3
-
PCl3
-
H2O
-
HCN
-
C2H4
-
C2H2
-
AlCl3
-
CH3+
-
H2SO4
-
SF6
-
SF4
-
XeF4
-
PCl6-
Additional Problems
-
Draw the Lewis structures of the following species, with all lone pairs shown.
-
Draw and name the electron pair geometry and the molecular geometry.
|
MOLECULE |
Lewis Structure |
ELECTRON PAIR GEOMETRY |
MOLECULAR GEOMETRY |
|
1. O2 |
|||
|
2. NH4+ |
|||
|
3. CO2 |
|||
|
4. CHCl3 |
|||
|
5. H3O+ |
|||
|
6. SO2 |
Summary for Lewis Structures and Geometry
-
List the rules for drawing Lewis structure
-
Write the formula for calculating Formal Charge
-
For ionic compounds, what should you assume about the charge on the following atoms?
-
Na
-
K
-
Mg
-
Ca
-
-
For what atoms can you draw a Lewis structure with more than an octet?
-
How do you determine the electronic geometry from the Lewis structure?
-
What is the difference between electronic and molecular geometry?
Application Problem
Part A: Nickel Oxide
The demand for portable electronic devices has advanced research on rechargeable batteries such as lithium ion batteries. Recent research has focused on 3d metal oxides to serve as negative electrode materials due to their ability to provide larger charging capacities safely.Chen, Z., Xiao, A., Chen, Y., Zuo, C., Zhou, S., Li, L. Materials Research Bulletin, 2012, 47, 1987-1990.
-
Nickel oxide can be viewed as a face-centered array of oxygen ions. Draw them in the blank unit cell shown below.
-
The nickel ions are in all of the octahedral holes. Draw them as well. Clearly label which are Ni ions and which are O ions.
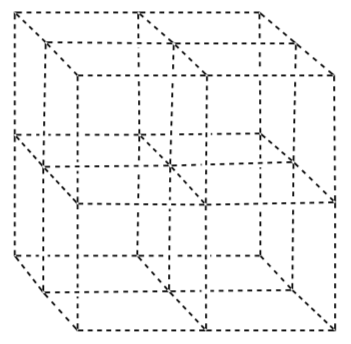
-
How many Ni ions are in the unit cell? (Show your work)
-
How many O ions are there in the unit cell? (Show your work)
-
Based on your unit cell, what is the empirical formula for nickel oxide?
-
What is the coordination number for the nickel ion?
-
Provide the full electron configurations for nickel and oxygen atoms.
Ni:
O:
-
Using the electron configuration of the oxygen atom, what charge would you predict for an oxide ion? Explain your answer.
-
What is the charge of the nickel ion? Explain your answer.
-
Draw an orbital filling diagram (arrows and energy levels) for the nickel ion.
-
Which would be larger, an oxide ion or an oxygen atom? Support your answer.
-
Which would be larger, an oxide ion or a lithium ion? Support your answer.
-
Which would have a higher lattice energy, nickel oxide or lithium oxide? Support your answer.
Part B: Lithium ions
The lithium ions in the electrochemical cell are provided by the electrolyte solution Li(PF6). The electrolyte solution facilitates the flow of ions between the positive and negative electrodes. The flow of ions provides the energy to power electronic devices.
-
Draw the Lewis structure for Li(PF6), including all non-zero formal charges.
-
Draw a perspective drawing using wedges and dashes.
-
What is the electron geometry around phosphorus?
Part C: Electrochemical performance (charging capacity)
-
Propose a reason why a porous electrode structure would improve electrochemical performance of a battery?

