Understanding Radiative Forcing: Activity
- Page ID
- 299347
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Intergovernmental Panel on Climate Change (IPCC) is an international group of scientists, which publish assessment reports on the effects of climate change (most recently in 2014). The most abundant cause of climate change is increased concentrations of greenhouse gases in the atmosphere. The effects of these gases are represented in their radiative forcing (RF): the increase or decrease in radiation at the Earth’s surface due to each type of greenhouse gas. Positive radiative forcings represent compounds that warm the atmosphere such as CO2. Some species such as sulfate aerosol actually cool the atmosphere and therefore have negative radiative forcings. Below is the radiative forcing for anthropogenic and natural drivers presented in the 2013 IPCC: Summary for Policymakers which is available at: http://www.ipcc.ch/report/ar5/syr/
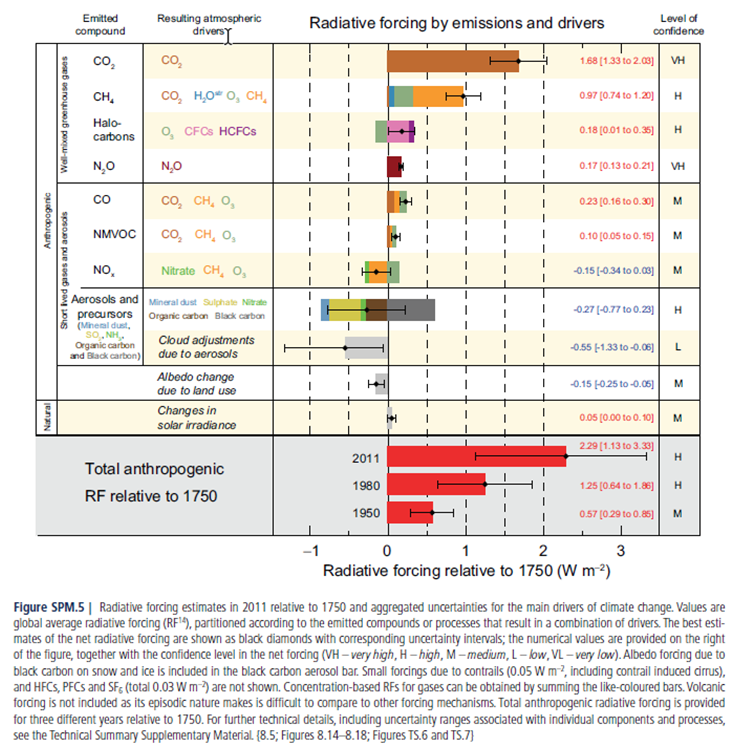
Carbon Dioxide
- Does CO2 warm or cool the atmosphere?
- Based on the figure what percentage of the total RF (2.29 W m-2) is due solely to CO2 emissions?
- CO2 is also listed under CH4, CO and NMVOC (non-methane volatile organic compounds) because they are oxidized to form CO2 (rows 2, 5 & 6). How do these indirect CO2 sources (brown bars on rows 2, 5 & 6) compare to direct CO2 emissions (row 1)?
Other Gas-Phase Species
- Halocarbons (such as CFCs) cause both negative and positive forcing. The positive forcing is because CFCs are greenhouse gases. What is the cause of the negative forcing?
- N2O and CO2 have very high levels of confidence, while all other factors have lower confidence. Why is this the case? (notice what’s different in the bar graphs about these two factors)
- NMVOCs are not greenhouse gases. Why are they listed as a factor in the anthropogenic RF? How is this related to urban air quality?
Aerosols & Other Factors
- What is the only aerosol component with a positive RF? What is a source of these?
- What aerosol component has the largest negative RF? What is a source of these?
- Why do clouds have a negative RF?
- Why is the RF from clouds so uncertain?
- Why aren’t volcanic emissions included in the chart? (hint: read the caption)
- Land use change has a negative RF. Why?
The image below may help. On the left is an image of Brazilian rainforests in 1975 and of the right is the same region with cleared rainforests in 2009. Photo courtesy of NASA: Images of Change https://climate.nasa.gov/images-of-c...lifornia-night

Synthesis
- If research could be done in order to improve our understanding of only one factor, which factor should be chosen to best reduce the uncertainty in our understanding of the anthropogenic RF?
- Using data from 2011, what is our best estimate for the ratio of anthropogenic-to-natural RF changes since 1750?
- Do the same as in #14 but determine what the lowest ratio of anthropogenic-to-natural RF?
(use the highest possible natural RF and lowest possible anthropogenic RF)
- In order to reduce anthropogenic forcing we could reduce emissions of greenhouse gases. Or we could increase emissions of any of the factors with negative RFs. However, this could have consequences. What are the detrimental consequences of increasing:
- CFC emissions
- NOx emissions
- SO2 emissions
- CFC emissions
Contributors and Attributions
- Andreas Beyersdorf, California State University San Bernadino (andreas.beyersdorf@csusb.edu)
- Sourced from the Analytical Sciences Digital Library


