Mass Spectra of THMs and Fluorobenzene
- Page ID
- 287614
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Qualitative Analysis via GC/MS
In-class assignment on understanding EI mass spectra
TRICHLOROMETHANE (AKA CHLOROFORM)
- Predict all possible elemental compositions and corresponding integer masses of your compound. Hint – look up a table of isotopic abundances of the elements and take into account all of the naturally occurring isotopes of the different elements in your compound.
Consider all permutations of elements and isotopes (12C or 13C, 1H or 2H, 35Cl or 37Cl)
- What is the most likely elemental composition of the M+1 ion (where M corresponds to the molecular ion)?
- What is the most likely elemental composition of the M+2 ion?
- What is the most likely elemental composition of the M+4 ion?
- Look up the EI mass spectrum of your assigned compound (http://webbook.nist.gov/chemistry/).
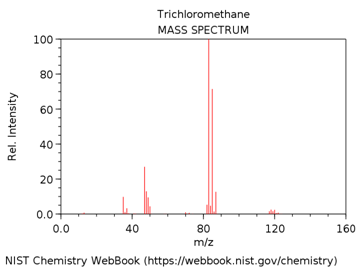
What is m/z value of the base peak in the mass spectrum?
What is the elemental composition of the ion corresponding to the base peak.
Write a balanced reaction showing the formation of this species.
Why is this ion the base peak? Hint – consider its stability.
DICHLOROBROMOMETHANE
- Predict all possible elemental compositions and corresponding integer masses of your compound. Hint – look up a table of isotopic abundances of the elements and take into account all of the naturally occurring isotopes of the different elements in your compound.
Consider all permutations of elements and isotopes (12C or 13C, 1H or 2H, 35Cl or 37Cl, 79Br or 81Br)
- What is the most likely elemental composition of the M+1 ion (where M corresponds to the molecular ion)?
- What is the most likely elemental composition of the M+2 ion?
- What is the most likely elemental composition of the M+4 ion?
- Look up the EI mass spectrum of your assigned compound (http://webbook.nist.gov/chemistry/).
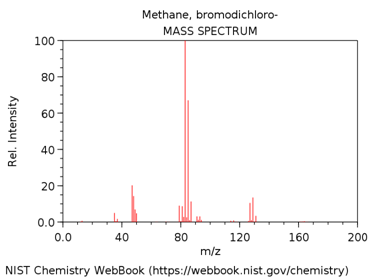
What is m/z value of the base peak in the mass spectrum?
What is the elemental composition of the ion corresponding to the base peak.
Write a balanced reaction showing the formation of this species.
Why is this ion the base peak? Hint – consider its stability.
CHLORODIBROMOMETHANE
- Predict all possible elemental compositions and corresponding integer masses of your compound. Hint – look up a table of isotopic abundances of the elements and take into account all of the naturally occurring isotopes of the different elements in your compound.
Consider all permutations of elements and isotopes (12C or 13C, 1H or 2H, 35Cl or 37Cl, 79Br or 81Br)
- What is the most likely elemental composition of the M+1 ion (where M corresponds to the molecular ion)?
- What is the most likely elemental composition of the M+2 ion?
- What is the most likely elemental composition of the M+4 ion?
- Look up the EI mass spectrum of your assigned compound (http://webbook.nist.gov/chemistry/).
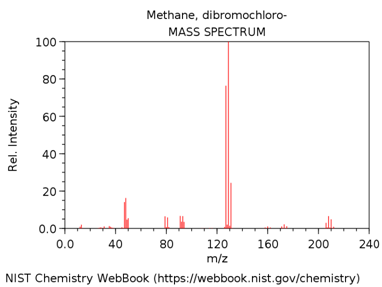
What is m/z value of the base peak in the mass spectrum?
What is the elemental composition of the ion corresponding to the base peak.
Write a balanced reaction showing the formation of this species.
Why is this ion the base peak? Hint – consider its stability.
TRIBROMOMETHANE (AKA BROMOFORM)
- Predict all possible elemental compositions and corresponding integer masses of your compound. Hint – look up a table of isotopic abundances of the elements and take into account all of the naturally occurring isotopes of the different elements in your compound.
Consider all permutations of elements and isotopes (12C or 13C, 1H or 2H, 79Br or 81Br)
- What is the most likely elemental composition of the M+1 ion (where M corresponds to the molecular ion)?
- What is the most likely elemental composition of the M+2 ion?
- What is the most likely elemental composition of the M+4 ion?
- Look up the EI mass spectrum of your assigned compound (http://webbook.nist.gov/chemistry/).
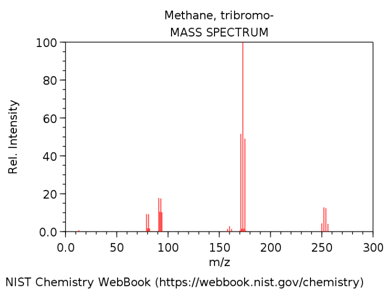
What is m/z value of the base peak in the mass spectrum?
What is the elemental composition of the ion corresponding to the base peak.
Write a balanced reaction showing the formation of this species.
Why is this ion the base peak? Hint – consider its stability.
FLUOROBENZENE
- What is the molecular formula of fluorobenzene?
- What is the average molecular weight of fluorobenzene?
- What is the nominal (integer) molecular weight (M) of fluorobenzene in which the C, H, and F atoms are all in the form of the most common isotopes?
- Predict all possible elemental compositions and corresponding integer masses of fluorobenzene. Hint – look up a table of isotopic abundances of the elements and take into account all of the naturally occurring isotopes of the different elements in your compound.
Consider all permutations of elements and isotopes (12C or 13C, 1H or 2H, 19F)
- What is the most likely elemental composition of the M+1 ion of fluorobenzene?
Hint - there must be an isotope in here - which is the most likely possibility?
- Look up the EI mass spectrum of fluorobenzene (http://webbook.nist.gov/chemistry/)
- What is m/z value of the base peak in the mass spectrum?
- Write a balanced reaction showing the formation of this species.
- Why is the molecular ion the base peak? Hint – consider its stability.
- What is m/z value of the base peak in the mass spectrum?
Contributors and Attributions
- Peter Palmer, San Francisco State University (palmer@sfsu.edu)
- Sourced from the Analytical Sciences Digital Library


