Fast Scan Cyclic Voltammetry
- Page ID
- 283141
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives: Fast Scan Cyclic Voltammetry
After completing this unit on Fast Scan Cyclic Voltammetry (FSCV), a student will be able to:
- Describe the role electrode size can play in cyclic voltammetry.
- Practice interpretation of cyclic voltammetry data analysis.
- Use the literature to analyze how microelectrodes are used in bioanalytical applications.
This is a three-part assignment. Parts 1 and 3 are intended to be done together in class, while part 2 is a reading guide to be used between classes.
This unit uses three articles from the literature:
- R. Mark Wightman, “Microvoltammetric Electrodes” Anal Chem, 1981, 53(9), 1125A-34A.
- A. C. Schmidt, X. Wang, Y. Zhu, and L. A. Sombers, “Carbon Nanotube Yarn Electrodes for Enhanced Detection of Neurotransmitter Dynamics in Live Brain Tissue,” ACS Nano, 2013, 7(9), 7864-7873.
- P.E.M. Phillips, G.D. Stuber, M.L.A.V. Heien, R.M. Wightman, and R.M. Carelli, “Subsecond Dopamine Release Promotes Cocaine Seeking,” Nature, 2003, 422, 614-618.
Name:_____________________________________
Part 1: Microelectrodes – Past and Future
Articles:
R. Mark Wightman, “Microvoltammetric Electrodes” Anal Chem, 1981, 53(9), 1125A-34A.
A. C. Schmidt, X. Wang, Y. Zhu, and L. A. Sombers, “Carbon Nanotube Yarn Electrodes for Enhanced Detection of Neurotransmitter Dynamics in Live Brain Tissue,” ACS Nano, 2013, 7(9), 7864-7873.
“Microvoltammetric Electrodes” Analytical Chemistry – R. Mark Wightman, August 1981
- Why would someone want to make small electrodes?
- Name examples of types of materials used to make micro-electrodes.
- Which material do you think is most popular?
- Where does one buy carbon fiber? What types of products is it used?
- Which material do you think is most popular?
- Fast-Scan Rates
- Compare the scan rates used in fast-scan current-voltage curves versus the ones we used in our electrochemistry lab. How much faster is Fast-Scan cyclic voltammetry?
- This article spends a significant amount of space describing how one can manipulate CV’s for electrodes with scan-speed. Draw the current versus potential diagrams comparing normal and extreme scan-speeds and between these two types of electrodes.
Macroelectrode
Microelectrode
Normal Scan Speeds
10-500 mV/s
10-500 mV/s
Extreme Scan Speeds
500-2000 µV/min
1-50 V/s
-
What role does the EC’ (catalysis) play in FSCV?
- Do microelectrodes experience the same catalytic effect as macroelectrodes? Why or why not?
- Why is this phenomenon important if you want to make measurements inside of a brain?
- Do microelectrodes experience the same catalytic effect as macroelectrodes? Why or why not?
- Compare the scan rates used in fast-scan current-voltage curves versus the ones we used in our electrochemistry lab. How much faster is Fast-Scan cyclic voltammetry?
- Applications of Microelectrodes
- Why/how do microelectrodes prevent tissue damage? Yes because they are small. How small is small? Why is small better? Be more specific.
- Electrodes “must be implanted in the brain and monitored for at least 8 h in most (in vivo, rat-brain) experiments.” Why is this important?
- One major failing with in vivo pulsed electrochemistry methods in the early 1980’s was the system’s inability to distinguish between molecular species. How does the author try and distinguish dopamine from interferents?
- Why/how do microelectrodes prevent tissue damage? Yes because they are small. How small is small? Why is small better? Be more specific.
“CNTy Electrodes for Enhanced Detection of NT Dynamics in Live brain Tissue” ACS Nano – Andreas Schmidt, Xin wang, Yuntian Zhu, Leslie Sombers, August 2013 (32 years later)
- In the 32 years since the discovery of carbon-fiber microelectrodes, how has the technology of these electrodes improved (I count four). Three of these methods have a very common theme between them; comment on their novelty and impact.
- Carbon nanotubes
- What’s a carbon nanotube?
- Why do they rock? (The authors list at least four reasons)
- What’s a carbon nanotube?
- Comparing CNTy and carbon fiber microelectrodes.
- The authors decide to compare their disk-shaped CNTy electrodes to cylindrical carbon-fiber microelectrodes, at first glance why might this be surprising?
- How do the authors justify this comparison?
- What is non-faradic current and why might it be a problem? How do the authors mitigate this issue?
- How do the CV’s of dopamine compare for the two types of electrodes compare? Is the reaction electrochemically reversible? Is the reaction chemically reversible?
- The authors decide to construct a calibration curve to further characterize the performance of their CNTy microelectrodes. They describe three improvements in these measurements with their electrodes. Describe and evaluate them.
- Consider Figures 2B and Table 1. In the table, summarize the anodic and cathodic peak currents and potentials for each electrode type. Do these two figures agree with one another?
Electrode Signals
Ipa
Ipc
Epa
Epc
C-fiber
Figure 2B
Table 1
CNTy
Figure 2B
Table 1
-
In Figures 5-7, concentration and scan-rate calibration curves are constructed to further characterize the performance of these microelectrodes, summarize these findings below.
Property
Carbon-fiber Electrode
CNTy electrode
Reason for improvement seen in CNTy electrodes
Sensitivity
Limit of Detection
Temporal Resolution
n.a.
Species Discrimination
- The authors decide to compare their disk-shaped CNTy electrodes to cylindrical carbon-fiber microelectrodes, at first glance why might this be surprising?
- Electrode Applications
- Why do the authors believe CNTy electrodes better at measuring dopamine?
- From the CV data, dopamine looks to be well measured with CNTy electrodes. How do other standards fair when using CNTy electrodes?
- How do CNTy electrodes fair being used in vivo electrochemistry experiments?
- Why do the authors believe CNTy electrodes better at measuring dopamine?
Name:__________________________________
Part 2: Microelectrode in vivo Electrochemistry Reading Guide
Articles:
P.E.M. Phillips, G.D. Stuber, M.L.A.V. Heien, R.M. Wightman, and R.M. Carelli, “Subsecond Dopamine Release Promotes Cocaine Seeking,” Nature, 2003, 422, 614-618.
A. C. Schmidt, X. Wang, Y. Zhu, and L. A. Sombers, “Carbon Nanotube Yarn Electrodes for Enhanced Detection of Neurotransmitter Dynamics in Live Brain Tissue,” ACS Nano, 2013, 7(9), 7864-7873.
Questions for both papers – Hint: many details can more readily be found at the end of the papers in the Methods sections
- Draw the half-cell reaction for the oxidation of dopamine to dopamine quinone.
- What reference electrodes were used in these studies?
- What scan rate(s) were used in these studies? Compare the papers.
- How many cyclic voltammograms (scans) were acquired per second (in Hz)?
- For a living system FSCV experiment, sketch the potential applied to the working electrode as a function of time. Include as much detail in your plot as possible.
- Consider the dopamine CVs done with carbon-fiber electrodes in Figure 1a of Nature and 2b of ACS Nano papers. Draw these CV’s and label the oxidation and reduction half-waves. Was the oxidation of dopamine reversible?
- In the experiments with 3D color plots, what plot would you obtain if you took a slice horizontally through the color plot? What would you obtain for a vertical slice?
Specific Questions for Nature, 2003, 422, 614-618.
- Look up any other unfamiliar words in the abstracts. Summarize each study in one complete sentence.
- How were the rats taught to self-administer cocaine? How was the cocaine delivered?
- What stimulus accompanied cocaine delivery?
- How did the authors correct for current from interferents, movement of the animal, and pH changes in the extracellular space?
- Complete the table below summarizing the means used to demonstrate that their signal came from dopamine release.
Experiment
How did this demonstrate the signal was from dopamine release?
anatomical
physiological
chemical
pharmacological
- Consider Figures 1-4. For each stimulus that was used to elicit dopamine release/drug-seeking behavior, estimate the concentration of dopamine released in nM and summarize any important findings about how the timing of the dopamine release related to the timing of the stimulus.
Figure
Stimulus/Conditions
Dopamine Release (nM)
Timing of Response
1
electrical stimulus train (24 pulses at 60 Hz)
2
rats were seeking and obtaining cocaine by lever press
3
audiovisual stimulus
(no cocaine)
4
electrical stimulus train (24 pulses at 60 Hz every 120 s)
n/a
Name:__________________________________
Part 3: Microelectrode in vivo Electrochemistry In-Class
Articles:
P.E.M. Phillips, G.D. Stuber, M.L.A.V. Heien, R.M. Wightman, and R.M. Carelli, “Subsecond Dopamine Release Promotes Cocaine Seeking,” Nature, 2003, 422, 614-618.
A. C. Schmidt, X. Wang, Y. Zhu, and L. A. Sombers, “Carbon Nanotube Yarn Electrodes for Enhanced Detection of Neurotransmitter Dynamics in Live Brain Tissue,” ACS Nano, 2013, 7(9), 7864-7873.
Specific Questions for: ACS Nano, 2013, 7(9), 7864-7873.
- Oxidation of dopamine (DA) at carbon electrodes in the presence of ascorbic acid (AA) has been shown to result in a catalytic oxidation of AA that regenerates DA. Why might this not be a problem for in vivo electrochemistry? Would this be less of a problem when using a yarn electrode?
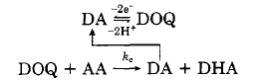
- Could yarn electrodes be used to detect dopamine, hydrogen peroxide, adenosine, serotonin, ascorbic acid, and DOPAC all at once in a living brain sample?
Questions for: Both Papers
- In these experiments, potentials are applied to the working electrode rather than an auxiliary electrode. Why might a counter/auxiliary electrode not be necessary in microelectrode experiments?
- Suggest some advantages of electrochemical detection of dopamine for these applications, compared to spectroscopic or mass spectrometric detection.
Specific Questions for Nature, 2003, 422, 614-618.
- In cocaine experiment, why was the cyclic voltammetry data alone not sufficient to identify the signal as coming from dopamine? Why might this signal not appear in the CNTy electrode experiment?
- Individuals recovering from drug addiction are often counseled to avoid “triggers”, including locations and situations in which they previously used drugs. Do the findings in this study support this advice? Why or why not? Cite a specific figure or figures in your answer.
Contributors and Attributions
- Nick Kuklinski, Furman University (nick.kuklinski@furman.edu)
- Part of this activity adapted Michelle Kovarik’s Electrochemistry Assignment from “Interpreting the Primary Literature” from the Analytical Sciences Digital Library (http://community.asdlib.org/activele...ry-literature/)
- Sourced from the Analytical Sciences Digital Library


