12.1: Side Reactions
- Page ID
- 213761
1. The reaction between NaBr and H2SO4 is an acid-base reaction. Bromide ion, which results from dissociation of NaBr in water, reacts with sulfuric acid to capture one of its acidic protons. Equilibrium in acid-base reactions favors formation of the weaker acid (HBr) and the weaker base (bisulfate ion).
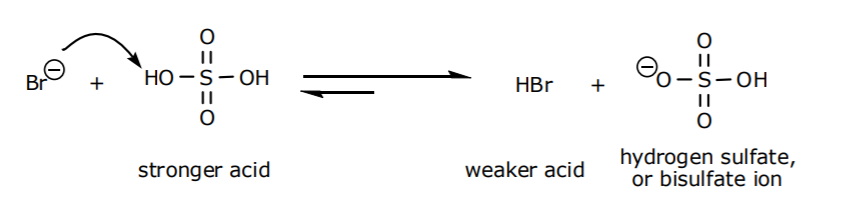
The first inorganic salt that forms as a byproduct is therefore NaHSO4. Remember, sodium is a expectator ion and serves as a counterion for any negative charges present in the medium.
2. Bromide can react with bisulfate ion in another acid base reaction to yield sulfate ion as another byproduct. Therefore Na2SO4 is the second inorganic salt that forms.
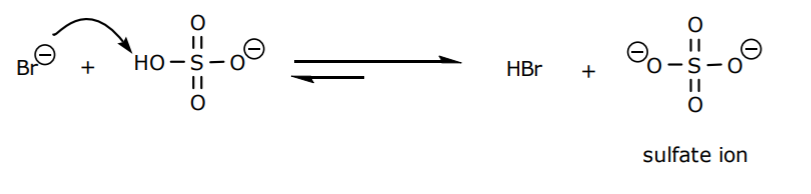
3. The total number of inorganic salts that can be present in the system is therefore three, namely NaBr, NaHSO4, and Na2SO4. Although they are soluble in water at room temperature, they may be less soluble in ice water and form crystalline precipitates.
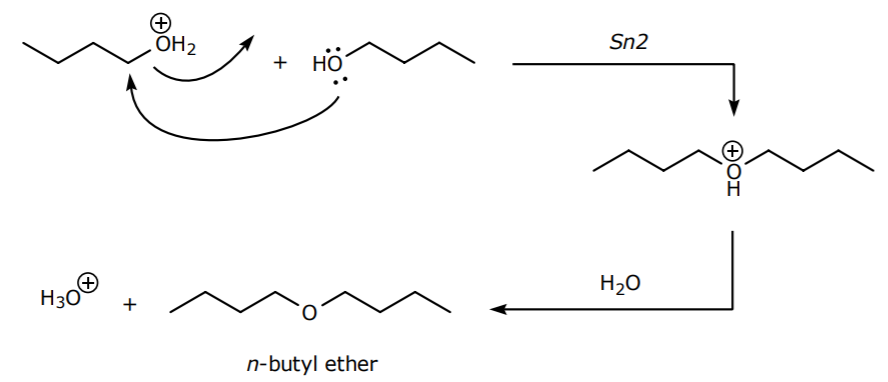
4. In part A (preparation of n-butyl bromide), another Sn2 side reaction leading to the formation of n-butyl ether is possible if the alcohol acts as the nucleophile.
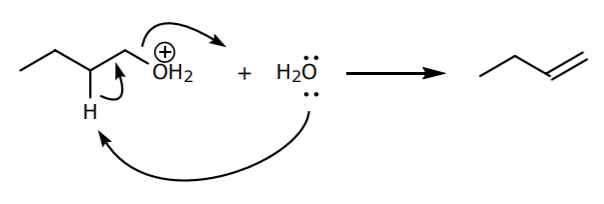
5. Finally, a less probable side reaction -an E2 reaction- can happen with water acting as a weak base. This reaction is less probable because E2 reactions normally require strong bases. However the reaction is still possible.