Experiment 2: Dyes - Methyl Orange
- Page ID
- 211969
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Background
Some types of organic structures give rise to color, while others do not. The partial structures necessary for color (unsaturated groups that can undergo π→π * and n→π * transitions) are called chromophores. Some common chromophores are:
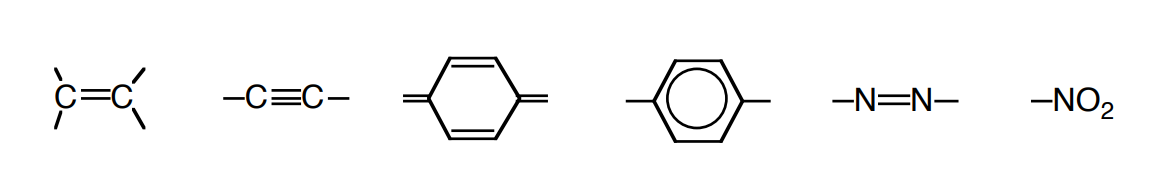
A dye is a colored organic compound that is used to impart color to an object or a fabric. Azo dyes, colored compounds containing the -N=N- group, are the largest and more important class of dyes. In azo-dyeing, the fabric is first impregnated with an aromatic compound activated toward electrophilic substitution, then is treated with a diazonium salt to form the dye (see reactions below).
An acid-base indicator is an organic compound that changes color with a change in pH. Methyl orange is a very common acid-base indicator, red in solutions that have pH values less than 3.2 and yellow in solutions with pH greater than 4.4. Indicators change color because the chromophoric system is changed by an acid base reaction (see below).
Materials:
- 50 mL Erlenmeyer flask
- 250 mL beaker
- 1.1 g of sulfanilic acid
- 13 mL of 2.5 % sodium carbonate (0.35 g of anhydrous sodium carbonate in 13 mL of water)
- 0.5 g of sodium nitrite
- 8 g of ice (approximately)
- 1.3 mL concentrated HCl 0.8 mL of N,N-dimethylaniline
- 0.7 mL of glacial acetic acid
- 8.5 mL of 3 M sodium hydroxide
- saturated sodium chloride solution
- 0.5 mL of 1 M sodium sulfate
- 1 M sulfuric acid
Safety: N,N-Dimethylanaline is combustible. Keep away from heat sources such as hot plates. Concentrated hydrochloric acid, glacial (concentrated) acetic acid are corrosive and will cause severe burns. Solutions of sodium hydroxide and sulfuric acid are corrosive and will cause burns. Avoid skin contact with diazonium salts. Some diazonium salts are explosive when dry. Always use in solution.
Procedure:
(a) Diazotization of Sulfanilic Acid
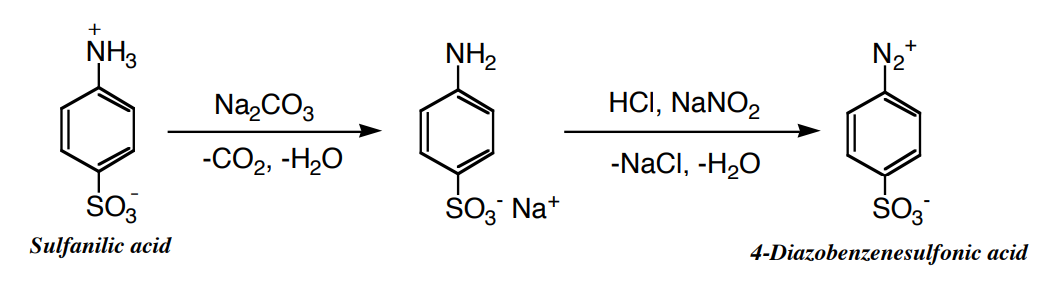
- In a 50 mL Erlenmeyer flask dissolve, by carefully boiling, 1.1 g of sulfanilic acid in 13 mL of 2.5 % sodium carbonate solution.
- Cool the solution under the tap and add 0.5 g of sodium nitrite, and stir until it is dissolved.
- Pour the solution into a 200 mL beaker containing about 8 g of ice (estimate, do not weigh) and 1.3 mL of concentrated hydrochloric acid. In a minute or two a powdery precipitate of the diazonium salt should separate, and the material is then ready for use.
(b) Methyl Orange (p-Sulfobenzeneazo-4-dimethylaniline sodium salt)
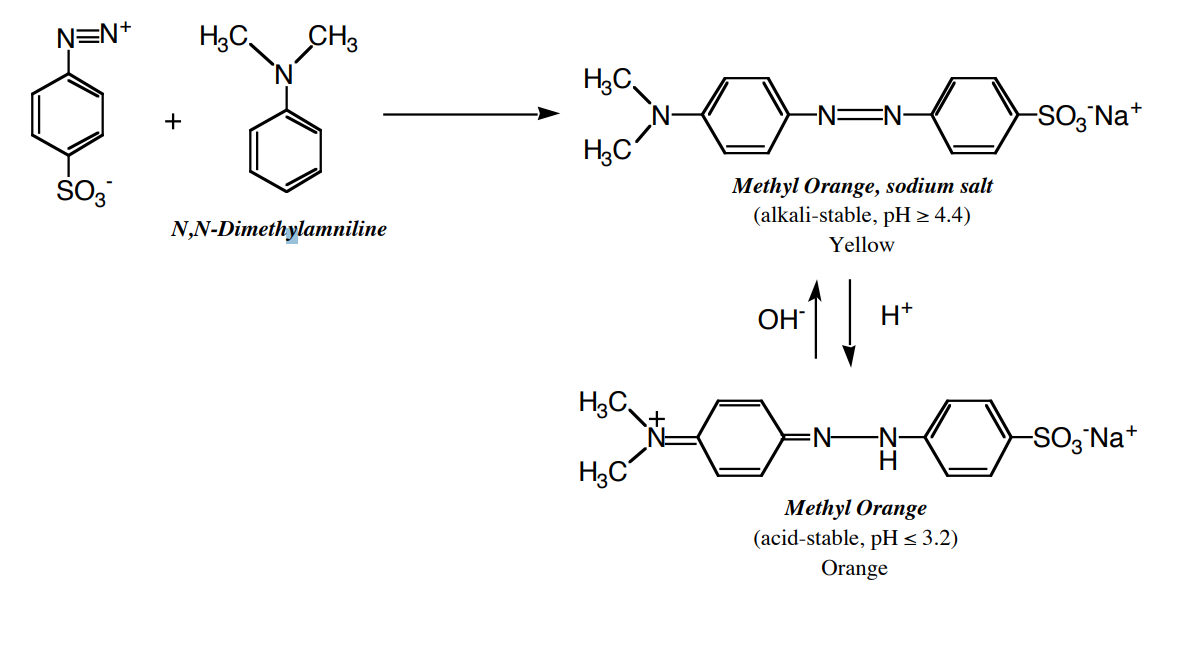
- In a test tube, thoroughly mix 0.8 mL of N,N-dimethylaniline and 0.7 mL of glacial acetic acid. Careful!!! Work under the hood!!!
- To the suspension prepared in part (a) add, with stirring, the solution of N,N-dimethylaniline acetate.
- Rinse the test tube with a small quantity of water and add it to the beaker.
- Stir and mix thorougly. Within a few minutes the red, acid-stable form of the dye should separate. A stiff paste should result in 5 to 10 minutes. (Note: if too much water is added it won't be stiff)
- Add 8.5 mL of 3 M sodium hydroxide solution to produce the orange sodium salt. (It will be foamy)
- Stir well and heat the mixture just to the boiling point.
- Place the beaker in a pan of ice and water, and allow the solution to cool undisturbed.
- Collect the product on a Büchner funnel, using saturated sodium chloride solution to rinse the flask and wash the filter cake. Use the house vacuum line (not the water aspirator). Be sure the filter paper fits properly and is wet (with distilled water) prior to filtration.
(c) Dyeing
- In a 30mL beaker, prepare a dye bath by dissolving 50 mg of Methyl Orange and 0.5 mL of 1 M sodium sulfate solution in 15 mL of water. Add 5 drops of 1 M sulfuric acid.
- Place a small piece of fabric in the bath at a temperature near the boiling point.
- After about 5 minutes, remove the fabric from the bath and let it cool. Rinse fabric in the marked rinse beakers prior to washing it thoroughly under running water and soap before drying it.
- Dip the cloth into dilute acid and then dilute base. Account for any changes.
Clean Up: Collect all waste in the designated containers.

