Experiment 1: Tollen's Test for Aldehydes and Ketones (The Silver Mirror)
- Page ID
- 211954
Background
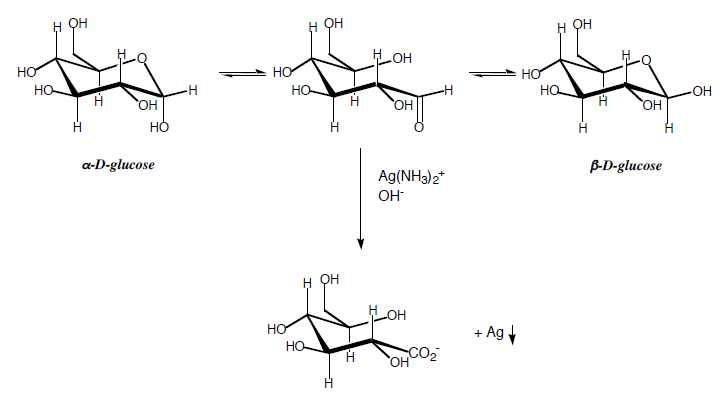
Silver (I) is reduced to silver metal by aldehydes, but not by ketones. Dextrose (D-glucose, a monosaccharide) is a polyhydroxyaldehyde which reduces silver.
In solution, either \(\alpha\)- or \(\beta\)-D-glucose (see figure II.2.1 below), undergoes a slow equilibrium with the open-chain form and with the other anomer. This equilibrium is called mutarotation.
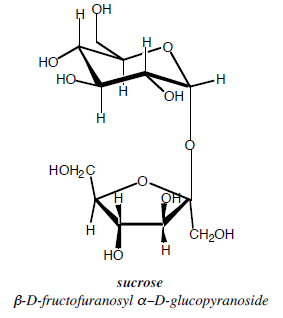
Sucrose on the other hand, is a disaccharide, a combination of glucose and fructose (figure II.2.2) inwhich both anomeric carbon atoms are used in theglycoside link. Therefore, sucrose in water is not in equilibrium with an aldehyde or keto form, and does no exhibit mutarotation and thus is not a reducing sugar. Sucrose however breaks down in the basic Tollen's Reagent and the resulting glucose will reduce the silver.
Materials:
Two 15mL test tubes.
Nitric Acid (HNO3)
Distilled water
0.1 M Silver Nitrate (AgNO3)
Concentrated Ammonia (NH3)
0.80 M Potassium Hydroxide (KOH)
0.25 M Glucose
0.25 M Sucrose
SAFETY
Concentrated nitric acid and concentrated ammonium hydroxide are highly corrosive and will cause severe burns. Vapors are harmful. Nitric acid is also a powerful oxidizing agent that may ignite or react explosively with many inorganic and organic substances. Potassium hydroxide solutions are irritating and may cause burns depending upon the concentration and length of exposure. Acetone is volatile and highly flammable. Do not use near a heat source. Silver nitrate solutions are irritating and oxidizing. Carry out the experiment in a well-ventilated hood.
Procedure:
- Rinse the test tubes with distilled water, nitric acid, water, acetone, and water in that order.
- Add 5 mL of 0.1 M silver nitrate to each test tube.
- Pour small amounts of ammonia to the silver nitrate solution and swirl until the solution is a brown color (muddy).
- Keep adding ammonia to this muddy mixture until it becomes colorless and clear again.
- Add about 2.5 mL of Potassium Hydroxide. If the solution becomes muddy (and remains after mixing), add more ammonia until the solution becomes colorless and clear again.
- To one test tube add 5 mL of glucose. To the other add 5 mL of sucrose. (Label test tubes first)
- At the same time, place the test tubes in a hot water bath.
- Swirl the tubes lightly and record your observations.
Clean-Up: The chemical waste should be disposed in the designated containers.
Discussion: Write the balanced chemical equations for all processes, including for the addition of ammonia and potassium hydroxide.

