Experiment 4: Equilibrium and Temperature - Complexes of Cobalt (II)
- Page ID
- 211949
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Background
In early photographic processes, salted papers were prepared by first floating the paper on a solution of \(KCl\) (or other halide) and then in the dark on a solution of \(AgNO_3\). \(AgCl\) is precipitated into the paper fiber structure. Today the silver halide is usually prepared in a gelatin layer. The silver halide is then exposed to light to capture an image. The image is obtained through the photochemical reduction of
\(Ag^+\) to \(Ag\) metal. The image can either be obtained through very long exposure times or a "latent" image can be further developed through the use of developing agents. To remove excess, unreacted silver halide from the paper or gelatin layer it must be made soluble. This has been accomplished since the beginning of photography through the use of a "fixer" sodium thiosulfate \((Na_2S_2O_3)\).
This process relies heavily on solubility and precipitation processes governed by solubility equilibria (with equilibrium constants know as \(K_{sp}\).) as well as complex formation equilibria (with equilibrium constants represented by \(K_f\) or \(\beta\) ).
Consider the solubility of AgCl. What is the concentration of \(Ag^+\) in a saturated solution (i.e., there is a solid present)?
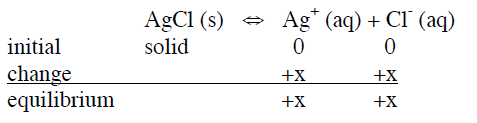
\[K_{sp} = \frac{[Ag^+][Cl^-]}{[AgCl]} = [Ag^+][Cl^-] = (x)(x) = 1.6 x 10^{-10}\nonumber\]
\[x = [Ag^+] = [Cl^-] = 1.3 x 10^{-5}\nonumber\]
The concentrations of the ions are very low in aqueous solution and whenever the concentrations of the ions exceeds \(1.3 x 10^{-5} M\) precipitation occurs and consequently unreacted silver halide is not readily removed by simple washing.
How to remove unwanted AgCl from the image?
\[\mathrm{Ag}^{+}(\mathrm{aq})+2 \mathrm{S}_{2} \mathrm{O}_{3}^{2-}(\mathrm{aq}) \Leftrightarrow\left[\mathrm{Ag}\left(\mathrm{S}_{2} \mathrm{O}_{3}\right)_{2}\right]^{3-}(\mathrm{aq})\nonumber\]
where \(\left[\mathrm{Ag}\left(\mathrm{S}_{2} \mathrm{O}_{3}\right)_{2}\right]^{3-}\) is very soluble
Combine the reactions:
\(\mathrm{AgCl}(\mathrm{s}) \Leftrightarrow \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{Cl}^{-}(\mathrm{aq})\) \(K_{sp}\)
\(\mathrm{Ag}^{+}(\mathrm{aq})+2 \mathrm{S}_{2} \mathrm{O}_{3}^{2-}(\mathrm{aq}) \Leftrightarrow\left[\mathrm{Ag}\left(\mathrm{S}_2\mathrm{O}_{3}\right)_2\right]^{3-}(\mathrm{aq})\) \(K_{f}\)
____________________________________________________
\({\mathrm{AgCl}(\mathrm{s})+2 \mathrm{S}_{2} \mathrm{O}_{3}^{2-}(\mathrm{aq}) \Leftrightarrow\left[\mathrm{Ag}\left(\mathrm{S}_{2} \mathrm{O}_{3}\right)_{2}\right]^{3-}(\mathrm{aq})+\mathrm{Cl}^{-} (\mathrm{aq})}\) \(K_{rxn} = K_{sp} x K_{f}\)
Coupling of two reactions permits pulling \(AgCl\) solid into the solution and with sufficient washing in water to remove the products, the light-sensitive silver halide is removed from the photographic image. In reality, there is a variety of soluble silver thiosulfate complexes formed.
To prepare a negative to be used for obtaining a cyanotype print later in the course a Polaroid Land camera and Polaroid Polapan 665P/N will be used. The chief advantage of this route to obtaining a negative is no darkroom is required to obtain a printable negative.
Materials
Polaroid 220 Land Camera
Polapan 665P/N (Polaroid Black and White Positive/Negative Instant Pack Film)
Sodium sulfite solution (\(Na_2S\))
Safety
Wear gloves when separating the film pack as the proprietary chemical mixture is caustic.
Procedure
You will be given instruction on the use of the camera to obtain an image and on the procedure for developing the instant pack film. The negatives are washed in a sodium sulfite solution and rinsed in water. A final rinse in a proprietary surfactant solution is used to minimize water spotting on the negative prior to hanging the negative to dry. To protect the positive image, the black and white print must be coated with the coater provided with the film.

