Experiment 2: Solubility Equilibrium - Precipitates and Complexes of Silver(I)
- Page ID
- 211948
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Background
The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent at a particular temperature. When an ionic solid such as silver chloride (\(AgCl\)) dissolves in water, it breaks up into ions that move apart from each other and become solvated by water molecules, and the following equilibrium is established:
\[AgCl(s) = Ag^+ (aq) + Cl^- (aq) \nonumber \]
The equilibrium constant for this reaction, or solubility product, \(K_{sp}\), is given by:
\[K_{sp} = [Ag^+][Cl^-] \nonumber \]
A simple relation exists between the solubility of an ionic compound (S) and its solubility product:
\[K_{sp} = S^2 \nonumber \]
It can be easily shown that for the general solubility equilibrium
\[ A_{n} B_{m}(s)=n A^{m+}(a q)+m B^{n-}(a q) \nonumber\]
the relation between the solubility and the solubility product is given by
\[K_{s p}=n^{n} m^{m} S^{n+m}\nonumber\]
The formation of coordination complexes can have a large effect on the solubility of a compound in water. Silver chloride is only very weakly soluble in water, but addition of ammonia \((NH_3)\) to the solution allows the complex ion \(\mathrm{Ag}\left(\mathrm{NH}_{3}\right)_{2}^{+}\) to form:
\[A g C l(s)+2 N H_{3}(a q)=A g\left(N H_{3}\right)_{2}^{+}(a q)+C l^{-}(a q)\nonumber\]
This greatly increases the solubility of the silver chloride.
Materials
1.0 mL of 0.1M silver nitrate \((AgNO3)\)
0.1M sodium bicarbonate \((NaHCO3)\)
0.1M sodium hydroxide \((NaOH)\)
0.1M sodium chloride \((NaCl)\)
5.0M ammonia \((NH3)\) (ammonium hydroxide, \(NH4OH\))
0.1M sodium bromide \((NaBr)\)
0.1M sodium thiosulfate \((Na2S2O3)\)
0.1M potassium iodide \((KI)\)
0.1M sodium sulfide \((Na2S)\)
50mL Beaker
Magnetic stirrer with stirring bar
Safety
Sulfide salts, their solutions and hydrogen sulfide gas, which smells like rotten eggs at low concentration and is produced by reaction of sulfides with acids, are all poisonous. Avoid adding any acid to a solution containing sulfide salts. Silver nitrate solutions are light sensitive, irritating and oxidizing. Ammonium hydroxide is corrosive and highly irritating upon inhalation.
Procedure
- Place a 50mL beaker containing 20 mL of distilled water and a magnetic stirring bar on the
magnetic stirrer, and adjust the rate of stirring so it is vigorous but not turbulent. - Add about 1 mL of \(AgNO_3\) solution and allow time for complete mixing.
- Add dropwise in sequence the following solutions until a change is clearly observed. This
will generally be 0.5-1.0 mL (10-20 drops): \(NaHCO_3\), \(NaOH\), \(NaCl\), \(NH_3\), \(NaBr\), \(Na_2S_2O_3\), \(KI\), and \(Na_2S\). - Write down your observations (color, state, etc.).
\[A g^{+}(a q) \stackrel{H C O_{3}^{-}}{\longrightarrow} A g_{2} C O_{3}(s) \stackrel{O H^{-}}{\longrightarrow} A g_{2} O(s) \stackrel{C l^-}{\longrightarrow} A g C l(s) \stackrel{N H_{3}}{\longrightarrow}\left[A g\left(N H_{3}\right)_{2}\right]^{+}\nonumber\]
\[\left[A g\left(N H_{3}\right)_{2}\right]^{+} \stackrel{B r^{-}}{\longrightarrow} A g B r(s) \stackrel{S_2 O_{1}^{2-}}{\longrightarrow}\left[A g\left(S_{2} O_{3}\right)_{2}\right]^{3-} \stackrel{I^-}{\longrightarrow} A g I(s) \stackrel{s^{2-}}{\longrightarrow} A g_{2} S(s)\nonumber\]
Clean-Up: Precipitate any silver still in solution by adding excess sodium sulfide.
Discussion
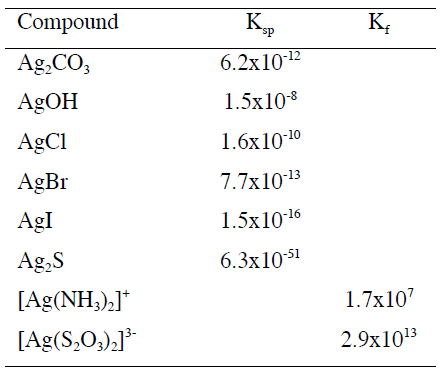
Silver forms a series of precipitates and complex ions with different solubilities and formation constants, respectively.
Interpret your results in terms of solubility constants, complex formation constants, and relative concentrations of reactants. How could you explain the different solubilities of the silver halides?