8: Acid, Bases and pH (Experiment)
- Page ID
- 95543
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To set up and show how to use a pH indicator
- To determine the pH of common solutions
- To understand pH differences of acids and bases
- To learn to use a laboratory pH meter
- To understand relationship between pH and H+ ion concentration
A pH value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. The “pH” is always written with a lowercase “p” and an uppercase “H”, which stands for “power of hydrogen.” pH values are related to hydrogen ion (\(\ce{H^+}\)) concentrations.
The mathematical relationship between pH and \(\ce{H^+}\) is described by the equation
\[pH = - \log(\ce{H^+})\]
There is an inverse relationship between pH and \(\ce{H^+}\) ion concentration (in brackets, expressed in units of molarity, M). As the \(\ce{H^+}\) concentration decreases, the pH value increases, and vice versa. When the pH value is a whole number (e.g. pH 7), the number is equal to the negative exponent of the \(\ce{H^+}\) ion concentration.
\[\text{pH value} = X \ce{->} [\ce{H^+}] = 10^{-X} M\]
So for pH 7, the \(\ce{H^+}\) ion concentration is 10-7 M.
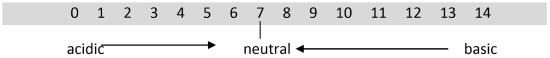
The pH values of everyday chemicals typically range from pH 0 to pH 14. Values between 0 and 7 indicate an acidic solution. Values between 7 and 14 indicate a basic solution. A pH of exactly 7 indicates that a solution is neutral, neither acidic or basic. Pure water is usually pH 7.
The pH scale is shown below.
The lower the pH value, the more acidic the solution; the higher the pH value, the more basic the solution. Basic solutions are also called alkaline solutions. It should be noted that the pH scale does extend beyond 0 and 14. Strong laboratory acids typically have pH values less than 0 (negative pH values) and strong laboratory bases typically have pH values greater than 14. Thus, they are considerably more dangerous.
The concept of pH is widely used in all areas of science including agriculture, biology, engineering and medicine. Many commercial products use pH as an advertisement tool, such as shampoo and water; more recently, food and drink of certain pH has been touted as more healthful.
A pH indicator is a substance that, when a small amount of it is added to a solution of unknown pH, will change its color. This is a way to determine pH of a solution visually. The indicator used in this lab will be obtained from a natural source, red cabbage. Cabbage indicator yields a particular color depending on the pH of the solution. pH indicators are a good way to easily and quickly show the approximate pH by color when compared to a standard. An everyday example where a pH indicator is used is for testing a water sample from a swimming pool.
While pH indicators are useful for qualitative purposes, when an exact quantitative value is needed, a pH meter is used. A laboratory pH meter typically has a special probe capped with a membrane that is sensitive to \(\ce{H^+}\) ion concentrations. The meter reading shows an exact pH value of the solution probed.
pH meters are used to measure pH values of water samples, such as determining acidity of rainwater samples. Rain water is contains dissolved carbon dioxide that produces a weakly acidic solution. Rain naturally has a pH between 5 and 6. The pH of rain in parts of the U.S. is less than pH 5, which is harmful to aquatic life and human health. This is acid rain.
Living organisms are very sensitive to the effects of acids and bases in their environment. An excess of \(\ce{H^+}\) or \(\ce{OH^-}\) can interfere with the functioning of biological molecules, especially proteins. Thus, in order to maintain homeostasis and survive, organisms must maintain a stable internal pH.
A buffer is a solution whose pH resists change on addition of small amounts of either an acid or a base. To be a good buffer, a solution should have a component that acts as a base (takes \(\ce{H^+}\) out of solution) and a component that acts as an acid (puts more \(\ce{H^+}\) into solution when there is an excess of \(\ce{OH^-}\)).
The buffering capacity of a solution is tested by adding small amounts of acid (for example, \(\ce{HCl}\)) and base (for example, \(\ce{NaOH}\)) and checking the pH after each addition. If the pH changes only slightly, the solution is a good buffer. Eventually its buffering capacity will be exhausted, however, and the pH will change dramatically.
Procedure
Materials and Equipment
400-mL beaker, ring stand, wire gauze, Bunsen burner, large test tubes, dropper pipet, stirring rod, wash bottle with distilled water, laboratory pH meter, 0.1 M acetic acid, 0.1 M \(\ce{NaC2H3O2}\), 0.1 M acetic acid (\(\ce{HC2H3O2}\)), 0.1 M hydrochloric acid (\(\ce{HCl}\)), 0.1 M sodium hydroxide (\(\ce{NaOH}\)), pH paper
Exercise appropriate caution when using the Bunsen burner.
Personal Protective Equipment (PPE) required: safety goggles, lab coat, closed-toe shoes Materials and Equipment
Experimental Procedure
Part A: Preparing pH indicator and pH standards - INSTRUCTOR DEMO
- Tear a few leaves of red cabbage into small pieces and place the leaves into a 500-mL beaker. Add about 500-mL of distilled water to this beaker. Make sure that all of the leaf pieces are completely submerged.
- Gently boil the mixture on heating plate until it appears dark purple in color (5-10 min). Turn off the heat and allow to cool (5 min).
- Add cabbage indicator solution to pH standard solutions, labeled 1-13. Students will record the colors of the pH standards.
- Each group will bring a small beaker to the front and take ~50 mL of the cabbage indicator back to their bench.
Part B: Qualitative Analysis for pH Values of Everyday Chemicals
- Obtain 10 large test tubes (clean, but may be wet). Label each test tube with the solutions to be tested.
- Pour about 3-mL of each solution into the appropriately labeled test tube.
- Using a dropper pipet, add an equal volume of cabbage indicator solution. If necessary, stir to mix with a clean stirring rod (rinse with distilled water between uses).
- Record the resulting color of the sample after mixed with the cabbage indicator. Compare this color with pH standards at the front of the laboratory to determine the pH of the sample. The color may be between the pH standard colors (e.g. green-blue instead of green or blue alone). For these, record the pH to 0.5 values (e.g. pH = 9.5 instead of 9 or 10).
Do not discard the contents in these test tubes as they will be used in the next section.
Part C: Quantitative Analysis for pH Values of Everyday Chemicals
- Plug the probe into one port on the side of the pH meter. Plug the AC adapter into the other side of the pH meter; plug the adapter into an electrical outlet. You should see a pH value reading.
- Prepare the probe to make pH measurements: remove from the storage bottle and thoroughly rinse the lower section of the probe with distilled water/wash bottle.
Note: Do not completely submerge the probe. The handle is not waterproof.
- Use the same ten test tubes containing samples from Part B. Or, complete steps 1 and 2 of Part B to obtain ten samples for analysis. Insert the pH probe directly into each test tube. SPECIAL CARE IS NEEDED WHEN INSERTING THE PROBES INTO THE TEST TUBES. The probes must NOT touch the glass rim of the test tubes or the pH blub can easily be broken and the probe destroyed.
- Record the pH value (to 0.01 pH) shown on the pH meter screen.
- After each pH measurement, the probe must be thoroughly rinsed with distilled water.
- When you are finished making measurements, rinse the probe with distilled water. Slide the cap onto the probe, and then screw the cap onto the storage bottle so the tip of the probe is immersed in the storage solution.
Part D: Effect of Buffers on pH
- Obtain 4 large test tubes. Label the test tubes A, B, C, and D.
- Add 10-mL of distilled water to tubes A and C.
- Add a 5-mL quantity of both 0.1 M H \(\ce{C2H3O2}\) (acetic acid) and 0.1 M \(\ce{NaC2H3O2}\) (sodium acetate) to tubes B and D. This mixture of acetic acid and sodium acetate is a buffer solution. Stir to mix completely.
- Using pH paper, determine the pH of the contents of each test tube (A-D). Use the stirring rod to dab a small drop of the solution to be tested onto a piece of pH paper. Then compare the color obtained to the pH scale on the instructor’s desk to determine the pH value. Record these pH values to 0.1
- Add 5 drops of 0.1 M (\ce{HCl}\) (hydrochloric acid) to test tubes A and B. Record the pH using pH paper.
- Add 5 drops of 0.1 M \(\ce{NaOH}\) (sodium hydroxide) to test tubes C and D. Record the pH using pH paper.
Lab Report: Acids, Bases and pH
Part A: Color of Red Cabbage Indicator with pH standards
|
pH standard |
Color with Cabbage Indicator |
|---|---|
|
1 |
|
|
2 |
|
|
3 |
|
|
4 |
|
|
5 |
|
|
6 |
|
|
7 |
|
|
8 |
|
|
9 |
|
|
10 |
|
|
11 |
|
|
12 |
Parts B and C: pH of Everyday Chemicals
|
Chemical |
Color with Indicator | Qualitative pH (to 0.5) | Acidic, Basic, or Neutral? |
Quantitative pH (to 0.01) |
|---|---|---|---|---|
|
soda |
||||
|
shampoo |
||||
|
ammonia cleaner |
||||
|
bleach |
||||
|
laundry detergent |
||||
|
lemon juice |
||||
|
vinegar |
||||
|
bottled water |
||||
|
0.1 M \(\ce{HCl}\) |
||||
|
0.1 M \(\ce{NaOH}\) |
Part D: Effect of Buffers on pH
|
Tube |
Contents |
Initial pH |
Chemical Added |
New pH |
pH change |
|---|---|---|---|---|---|
|
A |
Water |
\(\ce{HCl}\) |
|||
|
B |
Buffer solution |
\(\ce{HCl}\) |
|||
|
C |
Water |
\(\ce{NaOH}\) |
|||
|
D |
Buffer solution |
\(\ce{NaOH}\) |
Questions
- What is an acidic solution? What is a basic solution?
- What is an alkaline solution?
- What is a pH indicator? What are common uses of pH indicators?
- Write the mathematical equation that relates pH value and \(\ce{H^+}\) ion concentration:
- Circle correct choice:
Acids have (high OR low) pH, and (high OR low) \(\ce{H^+}\) ion concentration.
Bases have (high OR low) pH, and (high OR low) \(\ce{H^+}\) ion concentration.
- When the \(\ce{H^+}\) ion concentration is expressed in brackets [\(\ce{H^+}\)], what are the units of the for \(\ce{H^+}\) ion concentration?
- Does a solution with pH 10 have equal, less or more \(\ce{H^+}\) ions than of a solution with a pH 6? Calculate the [\(\ce{H^+}\)] for both solutions, include units in your answer:
- For pH 10, [\(\ce{H^+}\)] = _________________
- For pH 6, [\(\ce{H^+}\)] = _________________
- The two methods of determining pH values (pH indicator versus pH meter) should show similar pH values for those solutions. What was different?
- Explain why rain is naturally acidic, but not all rain is classified as “acid rain.
- Here are examples of what an individual might do to reduce acid rain. For each, explain the connection to production of acid rain.
- avoid running a washing machine with a small load
- add additional insulation on a home hot water heater
- walking instead of driving to work